SCZ 2018 - Brain_Frontal_Cortex_BA9
sheng Qian
2021-2-6
Last updated: 2022-05-19
Checks: 5 2
Knit directory: cTWAS_analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.7.0). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
The R Markdown file has unstaged changes. To know which version of the R Markdown file created these results, you’ll want to first commit it to the Git repo. If you’re still working on the analysis, you can ignore this warning. When you’re finished, you can run wflow_publish to commit the R Markdown file and build the HTML.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20211220) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Using absolute paths to the files within your workflowr project makes it difficult for you and others to run your code on a different machine. Change the absolute path(s) below to the suggested relative path(s) to make your code more reproducible.
| absolute | relative |
|---|---|
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/data/ | data |
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/code/ctwas_config.R | code/ctwas_config.R |
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version bcaadf3. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .Rhistory
Ignored: .ipynb_checkpoints/
Untracked files:
Untracked: G_list.RData
Untracked: Rplot.png
Untracked: SCZ_annotation.xlsx
Untracked: analysis/.ipynb_checkpoints/
Untracked: code/.ipynb_checkpoints/
Untracked: code/AF_out/
Untracked: code/Autism_out/
Untracked: code/BMI_S_out/
Untracked: code/BMI_out/
Untracked: code/Glucose_out/
Untracked: code/LDL_S_out/
Untracked: code/SCZ_2014_EUR_out/
Untracked: code/SCZ_2018_S_out/
Untracked: code/SCZ_2018_out/
Untracked: code/SCZ_2020_Single_out/
Untracked: code/SCZ_2020_out/
Untracked: code/SCZ_S_out/
Untracked: code/SCZ_out/
Untracked: code/T2D_out/
Untracked: code/ctwas_config.R
Untracked: code/mapping.R
Untracked: code/out/
Untracked: code/process_scz_2018_snps.R
Untracked: code/run_AF_analysis.sbatch
Untracked: code/run_AF_analysis.sh
Untracked: code/run_AF_ctwas_rss_LDR.R
Untracked: code/run_Autism_analysis.sbatch
Untracked: code/run_Autism_analysis.sh
Untracked: code/run_Autism_ctwas_rss_LDR.R
Untracked: code/run_BMI_analysis.sbatch
Untracked: code/run_BMI_analysis.sh
Untracked: code/run_BMI_analysis_S.sbatch
Untracked: code/run_BMI_analysis_S.sh
Untracked: code/run_BMI_ctwas_rss_LDR.R
Untracked: code/run_BMI_ctwas_rss_LDR_S.R
Untracked: code/run_Glucose_analysis.sbatch
Untracked: code/run_Glucose_analysis.sh
Untracked: code/run_Glucose_ctwas_rss_LDR.R
Untracked: code/run_LDL_analysis_S.sbatch
Untracked: code/run_LDL_analysis_S.sh
Untracked: code/run_LDL_ctwas_rss_LDR_S.R
Untracked: code/run_SCZ_2014_EUR_analysis.sbatch
Untracked: code/run_SCZ_2014_EUR_analysis.sh
Untracked: code/run_SCZ_2014_EUR_ctwas_rss_LDR.R
Untracked: code/run_SCZ_2018_analysis.sbatch
Untracked: code/run_SCZ_2018_analysis.sh
Untracked: code/run_SCZ_2018_analysis_S.sbatch
Untracked: code/run_SCZ_2018_analysis_S.sh
Untracked: code/run_SCZ_2018_ctwas_rss_LDR.R
Untracked: code/run_SCZ_2018_ctwas_rss_LDR_S.R
Untracked: code/run_SCZ_2020_Single_analysis.sbatch
Untracked: code/run_SCZ_2020_Single_analysis.sh
Untracked: code/run_SCZ_2020_Single_ctwas_rss_LDR.R
Untracked: code/run_SCZ_2020_analysis.sbatch
Untracked: code/run_SCZ_2020_analysis.sh
Untracked: code/run_SCZ_2020_ctwas_rss_LDR.R
Untracked: code/run_SCZ_analysis.sbatch
Untracked: code/run_SCZ_analysis.sh
Untracked: code/run_SCZ_analysis_S.sbatch
Untracked: code/run_SCZ_analysis_S.sh
Untracked: code/run_SCZ_ctwas_rss_LDR.R
Untracked: code/run_SCZ_ctwas_rss_LDR_S.R
Untracked: code/run_T2D_analysis.sbatch
Untracked: code/run_T2D_analysis.sh
Untracked: code/run_T2D_ctwas_rss_LDR.R
Untracked: code/wflow_build.R
Untracked: code/wflow_build.sbatch
Untracked: data/.ipynb_checkpoints/
Untracked: data/GO_Terms/
Untracked: data/PGC3_SCZ_wave3_public.v2.tsv
Untracked: data/SCZ/
Untracked: data/SCZ_2014_EUR/
Untracked: data/SCZ_2018/
Untracked: data/SCZ_2018_S/
Untracked: data/SCZ_2020/
Untracked: data/SCZ_S/
Untracked: data/Supplementary Table 15 - MAGMA.xlsx
Untracked: data/Supplementary Table 20 - Prioritised Genes.xlsx
Untracked: data/T2D/
Untracked: data/UKBB/
Untracked: data/UKBB_SNPs_Info.text
Untracked: data/gene_OMIM.txt
Untracked: data/gene_pip_0.8.txt
Untracked: data/mashr_Heart_Atrial_Appendage.db
Untracked: data/mashr_sqtl/
Untracked: data/scz_2018.RDS
Untracked: data/summary_known_genes_annotations.xlsx
Untracked: data/untitled.txt
Untracked: top_genes_32.txt
Untracked: top_genes_37.txt
Untracked: top_genes_43.txt
Untracked: top_genes_54.txt
Untracked: top_genes_81.txt
Untracked: z_snp_pos_SCZ.RData
Untracked: z_snp_pos_SCZ_2014_EUR.RData
Untracked: z_snp_pos_SCZ_2018.RData
Untracked: z_snp_pos_SCZ_2020.RData
Unstaged changes:
Deleted: analysis/BMI_S_results.Rmd
Modified: analysis/SCZ_2018_Brain_Amygdala_S.Rmd
Modified: analysis/SCZ_2018_Brain_Anterior_cingulate_cortex_BA24_S.Rmd
Modified: analysis/SCZ_2018_Brain_Caudate_basal_ganglia_S.Rmd
Modified: analysis/SCZ_2018_Brain_Cerebellar_Hemisphere_S.Rmd
Modified: analysis/SCZ_2018_Brain_Cerebellum_S.Rmd
Modified: analysis/SCZ_2018_Brain_Cortex_S.Rmd
Modified: analysis/SCZ_2018_Brain_Frontal_Cortex_BA9_S.Rmd
Modified: analysis/SCZ_2018_Brain_Hippocampus_S.Rmd
Modified: analysis/SCZ_2018_Brain_Hypothalamus_S.Rmd
Modified: analysis/SCZ_2018_Brain_Nucleus_accumbens_basal_ganglia_S.Rmd
Modified: analysis/SCZ_2018_Brain_Putamen_basal_ganglia_S.Rmd
Modified: analysis/SCZ_2018_Brain_Spinal_cord_cervical_c-1_S.Rmd
Modified: analysis/SCZ_2018_Brain_Substantia_nigra_S.Rmd
Modified: analysis/ttt.Rmd
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/SCZ_2018_Brain_Frontal_Cortex_BA9_S.Rmd) and HTML (docs/SCZ_2018_Brain_Frontal_Cortex_BA9_S.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | bcaadf3 | sq-96 | 2022-05-19 | update |
| html | bcaadf3 | sq-96 | 2022-05-19 | update |
| Rmd | be614ed | sq-96 | 2022-05-19 | update |
| html | be614ed | sq-96 | 2022-05-19 | update |
| Rmd | 7d08c9b | sq-96 | 2022-05-18 | update |
| html | 7d08c9b | sq-96 | 2022-05-18 | update |
| Rmd | 2749be9 | sq-96 | 2022-05-12 | update |
| html | 2749be9 | sq-96 | 2022-05-12 | update |
| html | 011327d | sq-96 | 2022-05-12 | update |
| Rmd | 6c6abbd | sq-96 | 2022-05-12 | update |
library(reticulate)
use_python("/scratch/midway2/shengqian/miniconda3/envs/PythonForR/bin/python",required=T)Weight QC
#number of imputed weights
nrow(qclist_all)[1] 21088#number of imputed weights by chromosome
table(qclist_all$chr)
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16
1945 1489 1242 813 857 1099 1218 761 872 971 1260 1129 427 730 701 875
17 18 19 20 21 22
1505 291 1506 706 43 648 #number of imputed weights without missing variants
sum(qclist_all$nmiss==0)[1] 18528#proportion of imputed weights without missing variants
mean(qclist_all$nmiss==0)[1] 0.8786INFO:numexpr.utils:Note: NumExpr detected 56 cores but "NUMEXPR_MAX_THREADS" not set, so enforcing safe limit of 8.finish
Attaching package: 'dplyr'The following objects are masked from 'package:stats':
filter, lagThe following objects are masked from 'package:base':
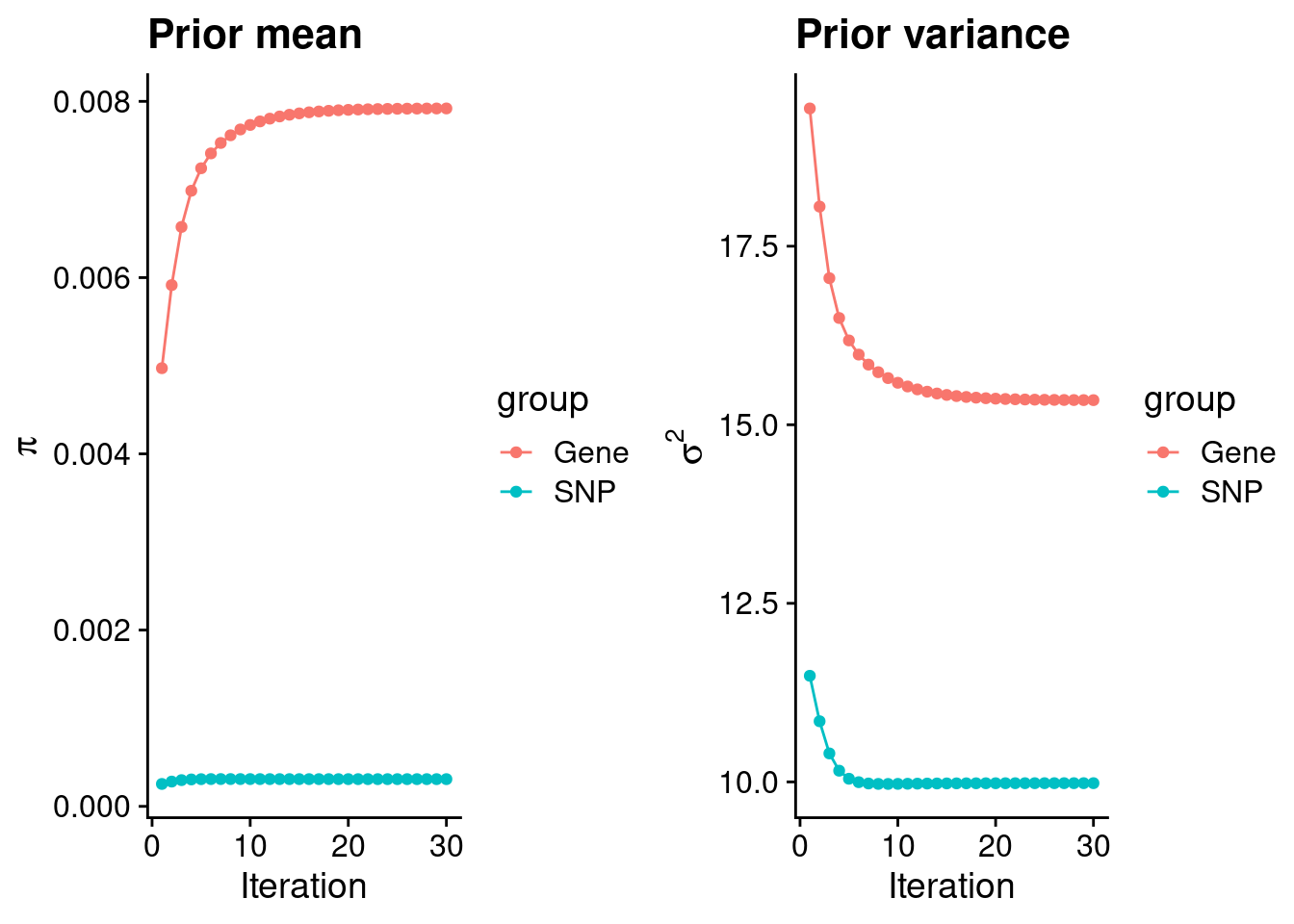
intersect, setdiff, setequal, unionCheck convergence of parameters
| Version | Author | Date |
|---|---|---|
| 2749be9 | sq-96 | 2022-05-12 |
gene snp
0.0079195 0.0003085 gene snp
15.345 9.982 [1] 105318[1] 7501 6309950 gene snp
0.008655 0.184514 [1] 0.02202 1.09251Genes with highest PIPs
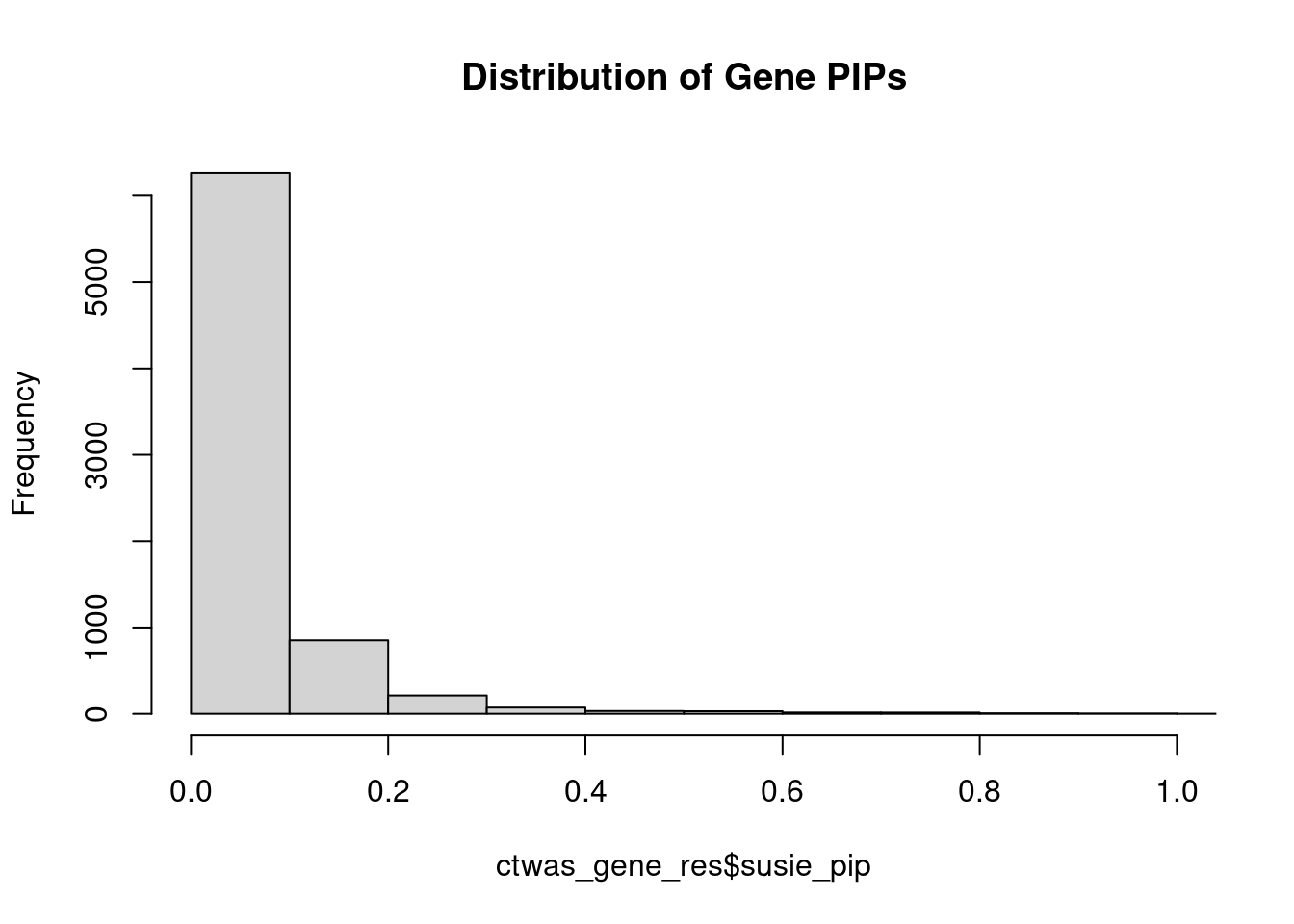
genename region_tag susie_pip mu2 PVE z num_intron
3525 LRP8 1_33 1.1351 34.30 0.0003112 4.820 6
791 BTN2A1 6_20 1.0285 157.11 0.0015234 -13.238 4
5082 PYROXD2 10_62 0.9822 23.05 0.0001954 -4.285 9
3424 LINC00320 21_6 0.9439 29.89 0.0002422 -5.336 4
719 BDNF 11_19 0.9220 23.77 0.0001901 -4.348 3
3360 LAMA5 20_36 0.9116 28.08 0.0001949 -4.299 14
1557 CRTAP 3_24 0.8882 20.38 0.0001502 3.929 3
652 B3GAT1 11_84 0.8763 22.16 0.0001522 4.287 7
6490 THAP8 19_25 0.8541 20.59 0.0001383 -3.802 2
6054 SNRPA1 15_50 0.8325 23.29 0.0001424 -3.925 6
4710 PLCB2 15_14 0.8308 25.63 0.0001382 4.470 5
5772 SF3B1 2_117 0.8056 47.59 0.0002835 -7.053 3
2595 GIGYF1 7_62 0.7930 28.66 0.0001691 5.266 2
3600 MAD1L1 7_3 0.7856 55.70 0.0003067 7.478 3
7044 VARS2 6_25 0.7827 95.93 0.0005580 -11.413 1
3576 LY6H 8_94 0.7826 22.30 0.0001256 -4.186 4
152 ACTR1B 2_57 0.7573 20.67 0.0001104 3.978 5
5829 SIPA1 11_36 0.7516 28.32 0.0001507 -4.893 2
6034 SMYD2 1_108 0.7427 23.47 0.0001207 3.952 3
1937 DPYSL3 5_86 0.7423 22.76 0.0001191 4.157 1
num_sqtl
3525 6
791 5
5082 10
3424 4
719 3
3360 21
1557 3
652 12
6490 4
6054 7
4710 5
5772 3
2595 2
3600 5
7044 1
3576 4
152 5
5829 2
6034 3
1937 1Genes with highest PVE
genename region_tag susie_pip mu2 PVE z num_intron
791 BTN2A1 6_20 1.0285 157.11 0.0015234 -13.238 4
439 APOM 6_26 0.4871 631.82 0.0014228 11.590 3
7043 VARS 6_26 0.4360 634.35 0.0011450 -11.620 1
7044 VARS2 6_25 0.7827 95.93 0.0005580 -11.413 1
3525 LRP8 1_33 1.1351 34.30 0.0003112 4.820 6
3600 MAD1L1 7_3 0.7856 55.70 0.0003067 7.478 3
5772 SF3B1 2_117 0.8056 47.59 0.0002835 -7.053 3
3424 LINC00320 21_6 0.9439 29.89 0.0002422 -5.336 4
2548 GATAD2A 19_15 0.6956 48.44 0.0002193 -6.668 4
2596 GIGYF2 2_137 0.7039 54.88 0.0002153 7.841 5
1816 DGKZ 11_28 0.6718 49.23 0.0002110 -7.216 2
5082 PYROXD2 10_62 0.9822 23.05 0.0001954 -4.285 9
3360 LAMA5 20_36 0.9116 28.08 0.0001949 -4.299 14
719 BDNF 11_19 0.9220 23.77 0.0001901 -4.348 3
2595 GIGYF1 7_62 0.7930 28.66 0.0001691 5.266 2
4135 NDRG4 16_31 0.6541 38.82 0.0001527 -6.343 4
6823 TSNARE1 8_93 0.7412 33.80 0.0001523 6.367 7
652 B3GAT1 11_84 0.8763 22.16 0.0001522 4.287 7
5829 SIPA1 11_36 0.7516 28.32 0.0001507 -4.893 2
1557 CRTAP 3_24 0.8882 20.38 0.0001502 3.929 3
num_sqtl
791 5
439 3
7043 1
7044 1
3525 6
3600 5
5772 3
3424 4
2548 4
2596 5
1816 2
5082 10
3360 21
719 3
2595 2
4135 4
6823 7
652 12
5829 2
1557 3Comparing z scores and PIPs
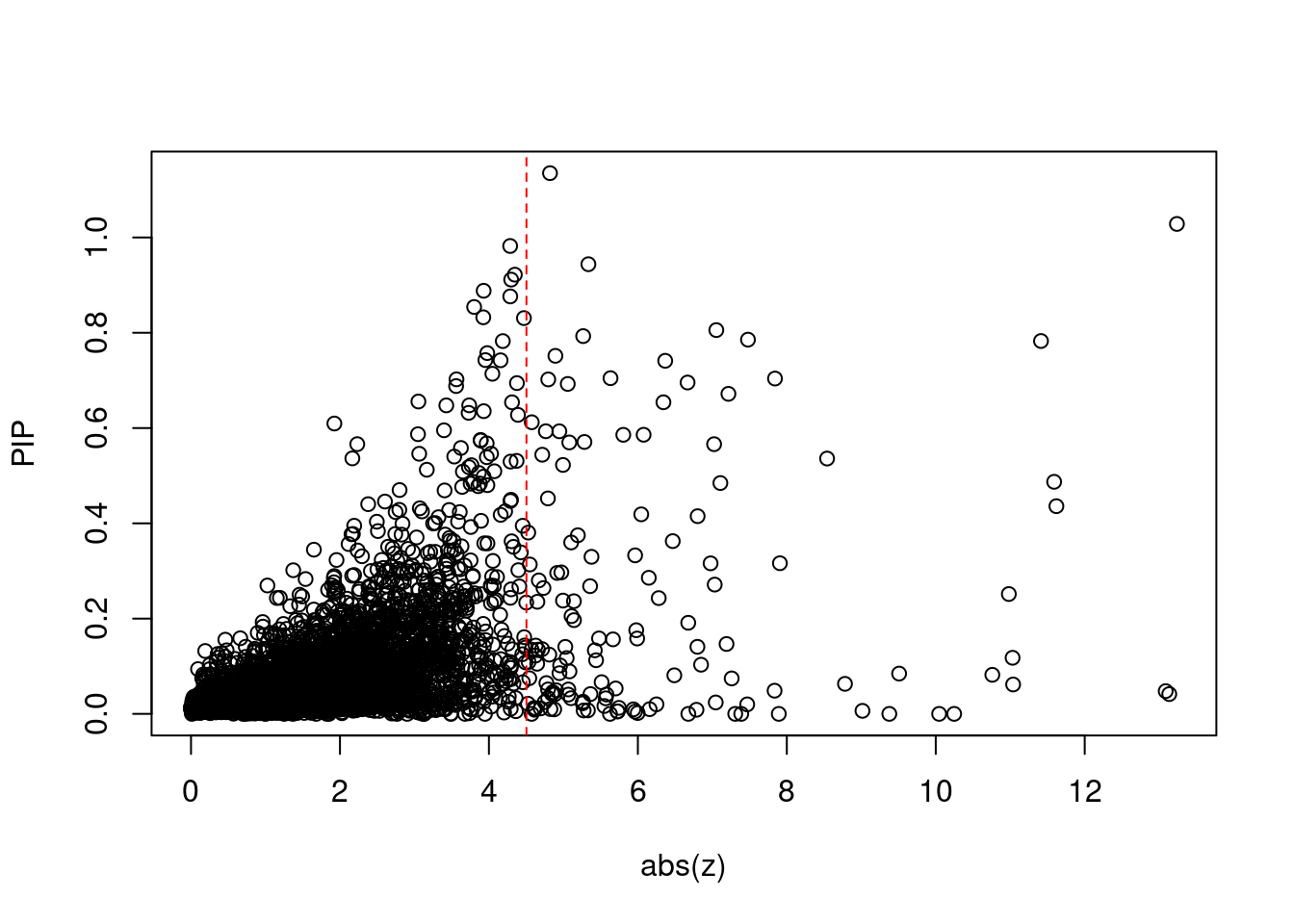
[1] 0.01866 genename region_tag susie_pip mu2 PVE z num_intron num_sqtl
791 BTN2A1 6_20 1.028e+00 157.11 1.523e-03 -13.238 4 5
7303 ZKSCAN3 6_22 4.155e-02 166.82 1.595e-06 -13.135 4 4
4612 PGBD1 6_22 4.774e-02 165.70 2.426e-06 -13.087 3 4
7043 VARS 6_26 4.360e-01 634.35 1.145e-03 -11.620 1 1
439 APOM 6_26 4.871e-01 631.82 1.423e-03 11.590 3 3
7044 VARS2 6_25 7.827e-01 95.93 5.580e-04 -11.413 1 1
561 ATAT1 6_24 6.167e-02 84.80 3.063e-06 11.039 1 1
928 C6orf136 6_24 1.177e-01 84.50 1.112e-05 -11.031 2 2
2438 FLOT1 6_24 2.516e-01 83.18 4.983e-05 10.981 6 7
793 BTN3A2 6_20 8.199e-02 103.10 2.442e-06 -10.759 4 5
667 BAG6 6_26 2.191e-09 504.13 2.299e-20 10.247 7 8
5369 RNF5 6_26 8.494e-13 470.42 3.223e-27 -10.045 1 1
1128 CCHCR1 6_25 8.454e-02 63.68 1.907e-06 9.508 14 20
2724 GPSM3 6_26 2.132e-14 419.92 1.812e-30 9.377 1 1
1768 DDR1 6_25 6.371e-03 62.32 2.402e-08 9.016 1 1
2897 HLA-DMA 6_27 6.303e-02 69.97 1.354e-06 8.781 5 7
4333 NT5C2 10_66 5.360e-01 50.29 1.300e-04 -8.541 10 14
535 AS3MT 10_66 3.162e-01 43.68 4.061e-05 7.907 4 4
3960 MSH5 6_26 0.000e+00 238.50 0.000e+00 -7.892 2 2
2596 GIGYF2 2_137 7.039e-01 54.88 2.153e-04 7.841 5 5GO enrichment analysis for genes with PIP>0.5
#number of genes for gene set enrichment
length(genes)[1] 71Uploading data to Enrichr... Done.
Querying GO_Biological_Process_2021... Done.
Querying GO_Cellular_Component_2021... Done.
Querying GO_Molecular_Function_2021... Done.
Parsing results... Done.
[1] "GO_Biological_Process_2021"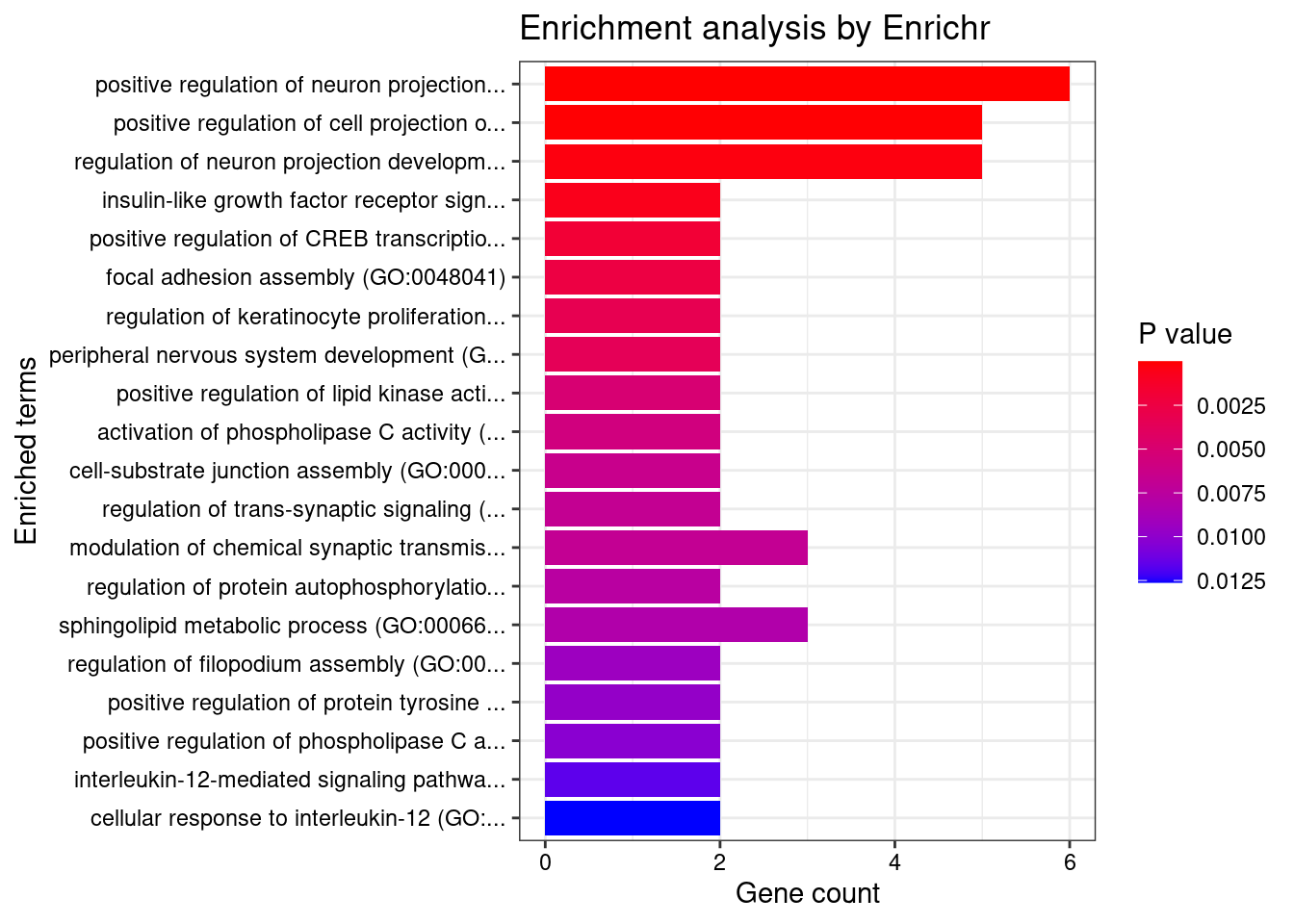
Term Overlap
1 positive regulation of neuron projection development (GO:0010976) 6/88
2 positive regulation of cell projection organization (GO:0031346) 5/117
Adjusted.P.value Genes
1 0.0003941 NDRG4;BDNF;DPYSL3;PRKD1;SERPINI1;LRP8
2 0.0170544 NDRG4;BDNF;DPYSL3;PRKD1;SERPINI1
[1] "GO_Cellular_Component_2021"
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)
[1] "GO_Molecular_Function_2021"
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)DisGeNET enrichment analysis for genes with PIP>0.5
Description FDR Ratio
52 Measles 0.05983 1/36
72 Schizophrenia 0.05983 10/36
209 Osteogenesis Imperfecta Type VII 0.05983 1/36
210 Maple Syrup Urine Disease, Type IA 0.05983 1/36
211 Familial encephalopathy with neuroserpin inclusion bodies 0.05983 1/36
217 HEMOLYTIC UREMIC SYNDROME, ATYPICAL, SUSCEPTIBILITY TO, 2 0.05983 1/36
221 MENTAL RETARDATION, AUTOSOMAL RECESSIVE 14 0.05983 1/36
224 MECKEL SYNDROME, TYPE 9 0.05983 1/36
228 MITOCHONDRIAL COMPLEX III DEFICIENCY, NUCLEAR TYPE 5 0.05983 1/36
236 SPASTIC PARAPLEGIA 45, AUTOSOMAL RECESSIVE 0.05983 1/36
BgRatio
52 1/9703
72 883/9703
209 1/9703
210 1/9703
211 1/9703
217 1/9703
221 1/9703
224 1/9703
228 1/9703
236 1/9703WebGestalt enrichment analysis for genes with PIP>0.5
Warning: replacing previous import 'lifecycle::last_warnings' by
'rlang::last_warnings' when loading 'hms'Loading the functional categories...
Loading the ID list...
Loading the reference list...
Performing the enrichment analysis...Warning in oraEnrichment(interestGeneList, referenceGeneList, geneSet, minNum =
minNum, : No significant gene set is identified based on FDR 0.05!NULLPIP Manhattan Plot
Warning: ggrepel: 26 unlabeled data points (too many overlaps). Consider
increasing max.overlaps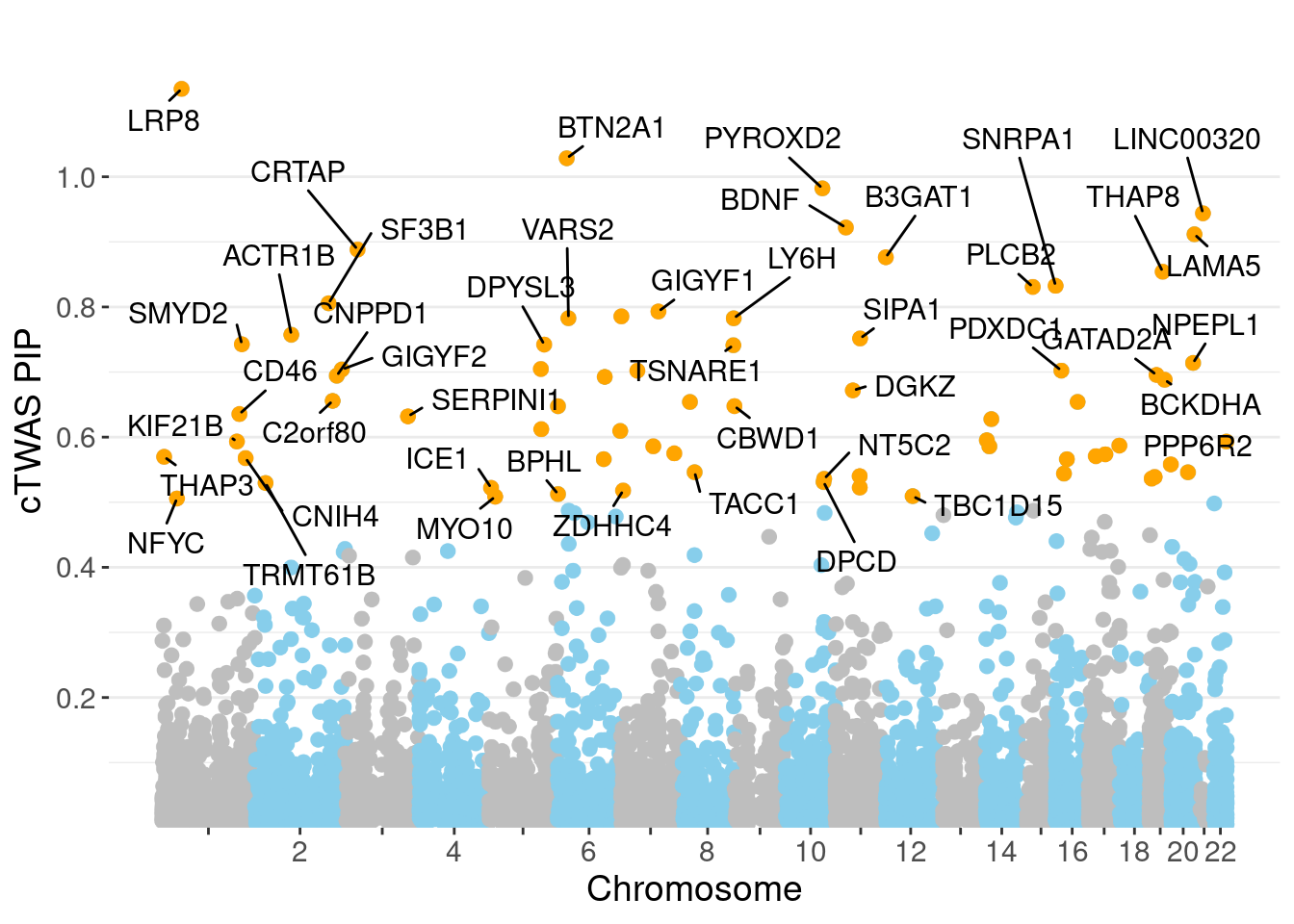
Sensitivity, specificity and precision for silver standard genes
#number of genes in known annotations
print(length(known_annotations))[1] 130#number of genes in known annotations with imputed expression
print(sum(known_annotations %in% ctwas_gene_res$genename))[1] 59#significance threshold for TWAS
print(sig_thresh)[1] 4.504#number of ctwas genes
length(ctwas_genes)[1] 12#number of TWAS genes
length(twas_genes)[1] 140#show novel genes (ctwas genes with not in TWAS genes)
ctwas_gene_res[ctwas_gene_res$genename %in% novel_genes,report_cols] genename region_tag susie_pip mu2 PVE z num_intron num_sqtl
652 B3GAT1 11_84 0.8763 22.16 0.0001522 4.287 7 12
719 BDNF 11_19 0.9220 23.77 0.0001901 -4.348 3 3
1557 CRTAP 3_24 0.8882 20.38 0.0001502 3.929 3 3
3360 LAMA5 20_36 0.9116 28.08 0.0001949 -4.299 14 21
4710 PLCB2 15_14 0.8308 25.63 0.0001382 4.470 5 5
5082 PYROXD2 10_62 0.9822 23.05 0.0001954 -4.285 9 10
6054 SNRPA1 15_50 0.8325 23.29 0.0001424 -3.925 6 7
6490 THAP8 19_25 0.8541 20.59 0.0001383 -3.802 2 4#sensitivity / recall
print(sensitivity) ctwas TWAS
0.02308 0.13077 #specificity
print(specificity) ctwas TWAS
0.9988 0.9835 #precision / PPV
print(precision) ctwas TWAS
0.2500 0.1214
sessionInfo()R version 4.1.0 (2021-05-18)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Scientific Linux 7.4 (Nitrogen)
Matrix products: default
BLAS/LAPACK: /software/openblas-0.3.13-el7-x86_64/lib/libopenblas_haswellp-r0.3.13.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] readxl_1.4.0 forcats_0.5.1 stringr_1.4.0 purrr_0.3.4
[5] readr_1.4.0 tidyr_1.1.3 tidyverse_1.3.1 tibble_3.1.7
[9] WebGestaltR_0.4.4 disgenet2r_0.99.2 enrichR_3.0 cowplot_1.1.1
[13] ggplot2_3.3.5 dplyr_1.0.7 reticulate_1.25 workflowr_1.7.0
loaded via a namespace (and not attached):
[1] fs_1.5.0 lubridate_1.7.10 doParallel_1.0.16 httr_1.4.2
[5] rprojroot_2.0.2 tools_4.1.0 backports_1.2.1 doRNG_1.8.2
[9] bslib_0.2.5.1 utf8_1.2.1 R6_2.5.0 vipor_0.4.5
[13] DBI_1.1.1 colorspace_2.0-2 withr_2.4.2 ggrastr_1.0.1
[17] tidyselect_1.1.1 processx_3.5.2 curl_4.3.2 compiler_4.1.0
[21] git2r_0.28.0 rvest_1.0.0 cli_3.0.0 Cairo_1.5-15
[25] xml2_1.3.2 labeling_0.4.2 sass_0.4.0 scales_1.1.1
[29] callr_3.7.0 systemfonts_1.0.4 apcluster_1.4.9 digest_0.6.27
[33] rmarkdown_2.9 svglite_2.0.0 pkgconfig_2.0.3 htmltools_0.5.1.1
[37] dbplyr_2.1.1 highr_0.9 rlang_1.0.2 rstudioapi_0.13
[41] jquerylib_0.1.4 farver_2.1.0 generics_0.1.0 jsonlite_1.7.2
[45] magrittr_2.0.1 Matrix_1.3-3 ggbeeswarm_0.6.0 Rcpp_1.0.7
[49] munsell_0.5.0 fansi_0.5.0 lifecycle_1.0.0 stringi_1.6.2
[53] whisker_0.4 yaml_2.2.1 plyr_1.8.6 grid_4.1.0
[57] ggrepel_0.9.1 parallel_4.1.0 promises_1.2.0.1 crayon_1.4.1
[61] lattice_0.20-44 haven_2.4.1 hms_1.1.0 knitr_1.33
[65] ps_1.6.0 pillar_1.7.0 igraph_1.2.6 rjson_0.2.20
[69] rngtools_1.5 reshape2_1.4.4 codetools_0.2-18 reprex_2.0.0
[73] glue_1.4.2 evaluate_0.14 getPass_0.2-2 modelr_0.1.8
[77] data.table_1.14.0 png_0.1-7 vctrs_0.3.8 httpuv_1.6.1
[81] foreach_1.5.1 cellranger_1.1.0 gtable_0.3.0 assertthat_0.2.1
[85] xfun_0.24 broom_0.7.8 later_1.2.0 iterators_1.0.13
[89] beeswarm_0.4.0 ellipsis_0.3.2 here_1.0.1