SCZ - Brain Spinal cord cervical c-1
sheng Qian
2021-2-6
Last updated: 2022-03-14
Checks: 5 2
Knit directory: cTWAS_analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.7.0). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
The R Markdown is untracked by Git. To know which version of the R Markdown file created these results, you’ll want to first commit it to the Git repo. If you’re still working on the analysis, you can ignore this warning. When you’re finished, you can run wflow_publish to commit the R Markdown file and build the HTML.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20211220) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Using absolute paths to the files within your workflowr project makes it difficult for you and others to run your code on a different machine. Change the absolute path(s) below to the suggested relative path(s) to make your code more reproducible.
| absolute | relative |
|---|---|
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/data/ | data |
| /project2/xinhe/shengqian/cTWAS/cTWAS_analysis/code/ctwas_config.R | code/ctwas_config.R |
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 4c71b11. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .ipynb_checkpoints/
Ignored: data/AF/
Untracked files:
Untracked: Rplot.png
Untracked: analysis/.ipynb_checkpoints/
Untracked: analysis/SCZ_2014_EUR_Brain_Amygdala.Rmd
Untracked: analysis/SCZ_2014_EUR_Brain_Anterior_cingulate_cortex_BA24.Rmd
Untracked: analysis/SCZ_2014_EUR_Brain_Caudate_basal_ganglia.Rmd
Untracked: analysis/SCZ_2014_EUR_Brain_Cerebellar_Hemisphere.Rmd
Untracked: analysis/SCZ_2014_EUR_Brain_Cerebellum.Rmd
Untracked: analysis/SCZ_2014_EUR_Brain_Cortex.Rmd
Untracked: analysis/SCZ_2014_EUR_Brain_Frontal_Cortex_BA9.Rmd
Untracked: analysis/SCZ_2014_EUR_Brain_Hippocampus.Rmd
Untracked: analysis/SCZ_2014_EUR_Brain_Hypothalamus.Rmd
Untracked: analysis/SCZ_2014_EUR_Brain_Nucleus_accumbens_basal_ganglia.Rmd
Untracked: analysis/SCZ_2014_EUR_Brain_Putamen_basal_ganglia.Rmd
Untracked: analysis/SCZ_2014_EUR_Brain_Spinal_cord_cervical_c-1.Rmd
Untracked: analysis/SCZ_2014_EUR_Brain_Substantia_nigra.Rmd
Untracked: analysis/SCZ_2020_Brain_Cortex.Rmd
Untracked: analysis/SCZ_2020_Brain_Frontal_Cortex_BA9.Rmd
Untracked: analysis/SCZ_2020_Brain_Hypothalamus.Rmd
Untracked: analysis/SCZ_2020_Brain_Putamen_basal_ganglia.Rmd
Untracked: analysis/SCZ_Cross_Tissue_Analysis.Rmd
Untracked: code/.ipynb_checkpoints/
Untracked: code/AF_out/
Untracked: code/Autism_out/
Untracked: code/BMI_S_out/
Untracked: code/BMI_out/
Untracked: code/Glucose_out/
Untracked: code/LDL_S_out/
Untracked: code/SCZ_2014_EUR_out/
Untracked: code/SCZ_2020_out/
Untracked: code/SCZ_S_out/
Untracked: code/SCZ_out/
Untracked: code/T2D_out/
Untracked: code/ctwas_config.R
Untracked: code/mapping.R
Untracked: code/out/
Untracked: code/run_AF_analysis.sbatch
Untracked: code/run_AF_analysis.sh
Untracked: code/run_AF_ctwas_rss_LDR.R
Untracked: code/run_Autism_analysis.sbatch
Untracked: code/run_Autism_analysis.sh
Untracked: code/run_Autism_ctwas_rss_LDR.R
Untracked: code/run_BMI_analysis.sbatch
Untracked: code/run_BMI_analysis.sh
Untracked: code/run_BMI_analysis_S.sbatch
Untracked: code/run_BMI_analysis_S.sh
Untracked: code/run_BMI_ctwas_rss_LDR.R
Untracked: code/run_BMI_ctwas_rss_LDR_S.R
Untracked: code/run_Glucose_analysis.sbatch
Untracked: code/run_Glucose_analysis.sh
Untracked: code/run_Glucose_ctwas_rss_LDR.R
Untracked: code/run_LDL_analysis_S.sbatch
Untracked: code/run_LDL_analysis_S.sh
Untracked: code/run_LDL_ctwas_rss_LDR_S.R
Untracked: code/run_SCZ_2014_EUR_analysis.sbatch
Untracked: code/run_SCZ_2014_EUR_analysis.sh
Untracked: code/run_SCZ_2014_EUR_ctwas_rss_LDR.R
Untracked: code/run_SCZ_2020_analysis.sbatch
Untracked: code/run_SCZ_2020_analysis.sh
Untracked: code/run_SCZ_2020_ctwas_rss_LDR.R
Untracked: code/run_SCZ_analysis.sbatch
Untracked: code/run_SCZ_analysis.sh
Untracked: code/run_SCZ_analysis_S.sbatch
Untracked: code/run_SCZ_analysis_S.sh
Untracked: code/run_SCZ_ctwas_rss_LDR.R
Untracked: code/run_SCZ_ctwas_rss_LDR_S.R
Untracked: code/run_T2D_analysis.sbatch
Untracked: code/run_T2D_analysis.sh
Untracked: code/run_T2D_ctwas_rss_LDR.R
Untracked: code/wflow_build.R
Untracked: code/wflow_build.sbatch
Untracked: data/.ipynb_checkpoints/
Untracked: data/BMI/
Untracked: data/PGC3_SCZ_wave3_public.v2.tsv
Untracked: data/SCZ/
Untracked: data/SCZ_2014_EUR/
Untracked: data/SCZ_2020/
Untracked: data/SCZ_S/
Untracked: data/T2D/
Untracked: data/UKBB/
Untracked: data/UKBB_SNPs_Info.text
Untracked: data/gene_OMIM.txt
Untracked: data/gene_pip_0.8.txt
Untracked: data/mashr_Heart_Atrial_Appendage.db
Untracked: data/mashr_sqtl/
Untracked: data/summary_known_genes_annotations.xlsx
Untracked: data/untitled.txt
Unstaged changes:
Modified: analysis/SCZ_Brain_Amygdala.Rmd
Modified: analysis/SCZ_Brain_Anterior_cingulate_cortex_BA24.Rmd
Modified: analysis/SCZ_Brain_Caudate_basal_ganglia.Rmd
Modified: analysis/SCZ_Brain_Cerebellar_Hemisphere.Rmd
Modified: analysis/SCZ_Brain_Cerebellum.Rmd
Modified: analysis/SCZ_Brain_Cortex.Rmd
Modified: analysis/SCZ_Brain_Frontal_Cortex_BA9.Rmd
Modified: analysis/SCZ_Brain_Hippocampus.Rmd
Modified: analysis/SCZ_Brain_Hypothalamus.Rmd
Modified: analysis/SCZ_Brain_Nucleus_accumbens_basal_ganglia.Rmd
Modified: analysis/SCZ_Brain_Putamen_basal_ganglia.Rmd
Modified: analysis/SCZ_Brain_Spinal_cord_cervical_c-1.Rmd
Modified: analysis/SCZ_Brain_Substantia_nigra.Rmd
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
There are no past versions. Publish this analysis with wflow_publish() to start tracking its development.
Weight QC
#number of imputed weights
nrow(qclist_all)[1] 10130#number of imputed weights by chromosome
table(qclist_all$chr)
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20
974 719 585 395 492 585 485 379 386 397 581 591 215 338 351 444 595 160 773 303
21 22
121 261 #number of imputed weights without missing variants
sum(qclist_all$nmiss==0)[1] 7997#proportion of imputed weights without missing variants
mean(qclist_all$nmiss==0)[1] 0.7894Check convergence of parameters
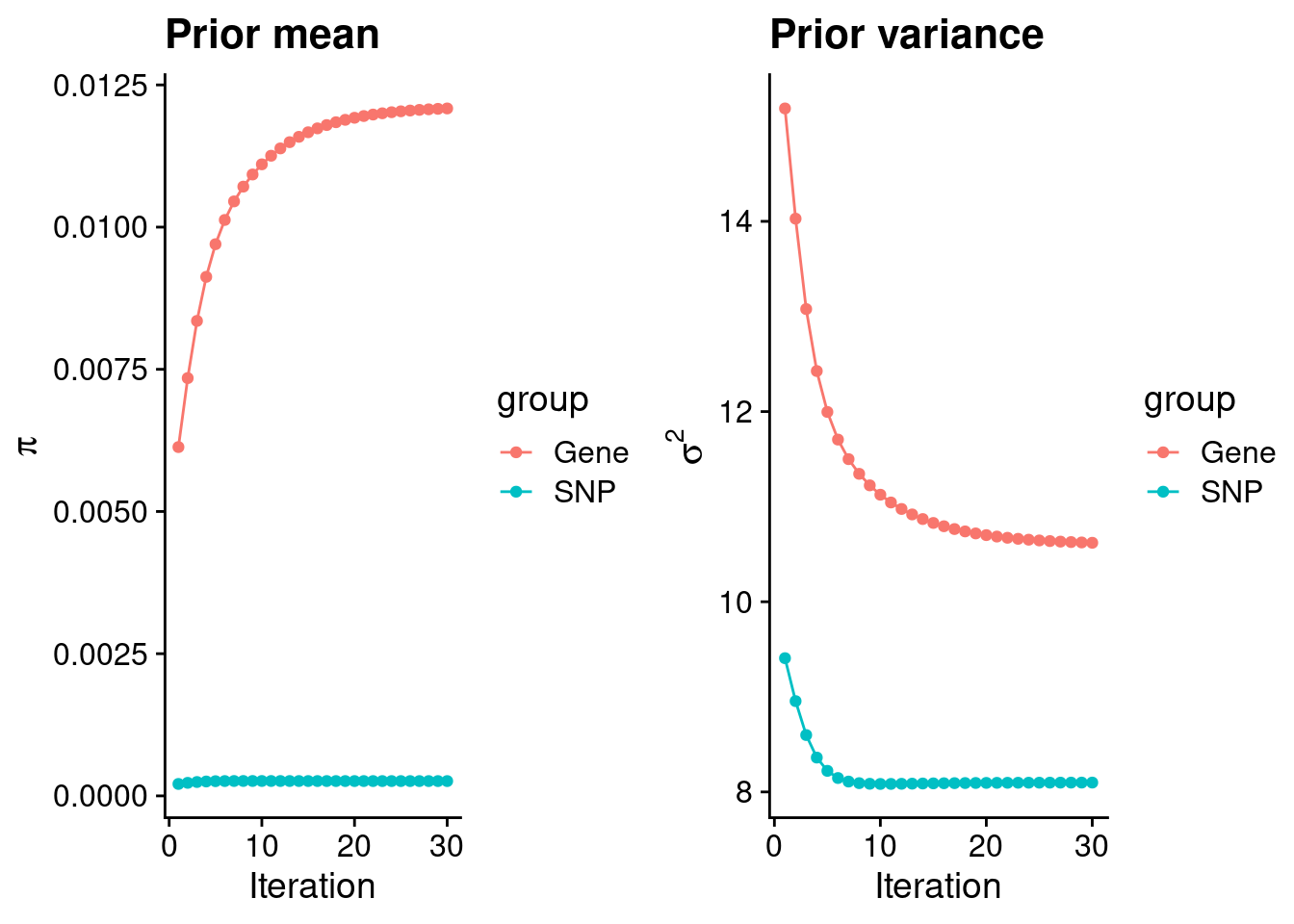
#estimated group prior
estimated_group_prior <- group_prior_rec[,ncol(group_prior_rec)]
names(estimated_group_prior) <- c("gene", "snp")
estimated_group_prior["snp"] <- estimated_group_prior["snp"]*thin #adjust parameter to account for thin argument
print(estimated_group_prior) gene snp
0.0120866 0.0002603 #estimated group prior variance
estimated_group_prior_var <- group_prior_var_rec[,ncol(group_prior_var_rec)]
names(estimated_group_prior_var) <- c("gene", "snp")
print(estimated_group_prior_var) gene snp
10.620 8.098 #report sample size
print(sample_size)[1] 77096#report group size
group_size <- c(nrow(ctwas_gene_res), n_snps)
print(group_size)[1] 10130 7352670#estimated group PVE
estimated_group_pve <- estimated_group_prior_var*estimated_group_prior*group_size/sample_size #check PVE calculation
names(estimated_group_pve) <- c("gene", "snp")
print(estimated_group_pve) gene snp
0.01687 0.20105 #compare sum(PIP*mu2/sample_size) with above PVE calculation
c(sum(ctwas_gene_res$PVE),sum(ctwas_snp_res$PVE))[1] 0.07211 1.72482Genes with highest PIPs
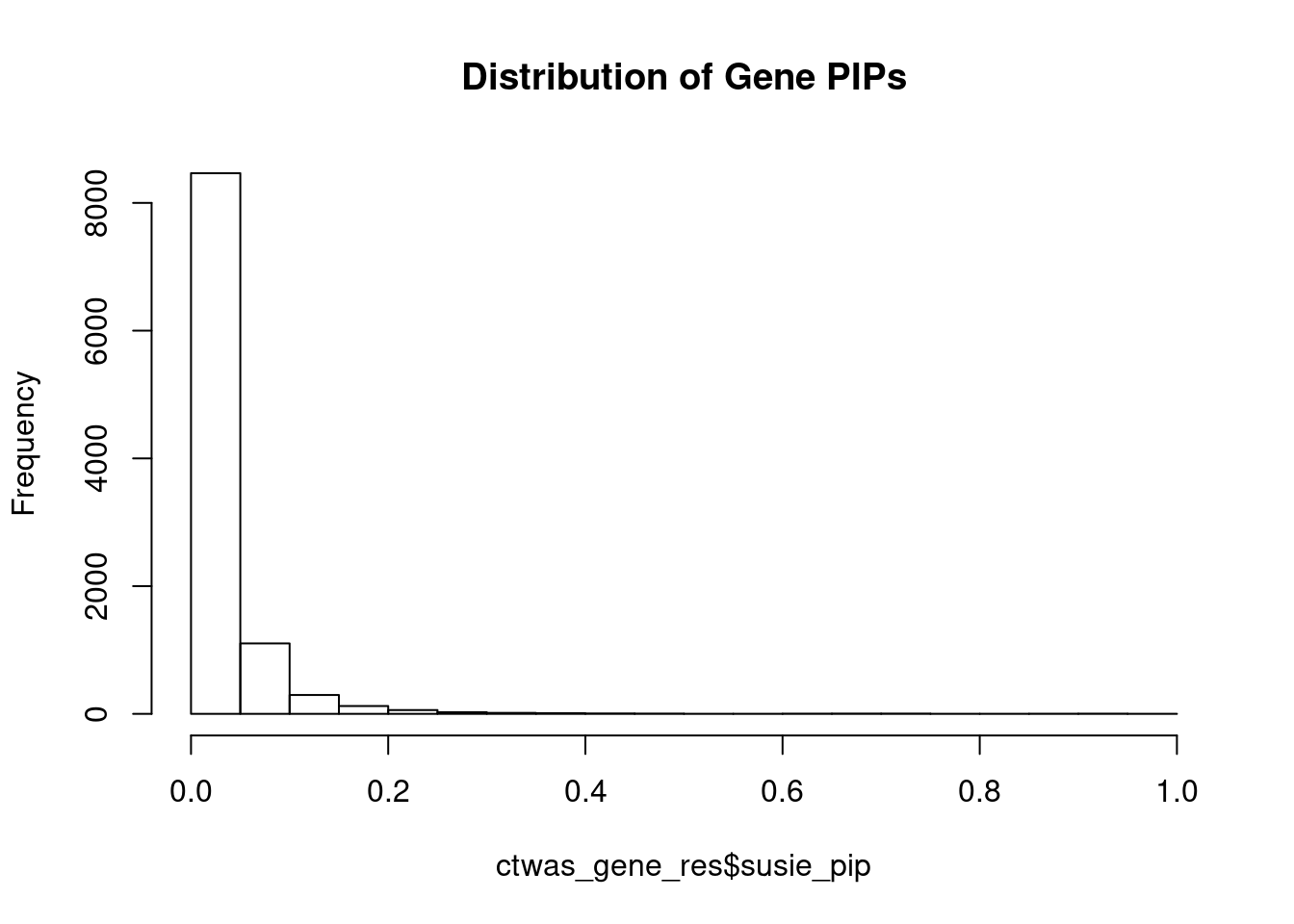
genename region_tag susie_pip mu2 PVE z num_eqtl
10447 ZNF823 19_10 0.9812 29.69 0.0003779 5.485 1
5935 ARFGAP2 11_29 0.9426 25.52 0.0003120 4.839 1
3025 MAP7D1 1_22 0.9221 25.98 0.0003107 5.058 1
2890 SF3B1 2_117 0.9090 45.13 0.0005321 6.784 1
11504 AC012074.2 2_15 0.8675 21.80 0.0002452 4.457 2
8557 MAP3K11 11_36 0.8541 22.88 0.0002535 -4.409 1
104 ELAC2 17_11 0.8446 21.79 0.0002387 4.518 1
3216 HSDL2 9_57 0.7845 22.13 0.0002251 4.378 1
2823 LMAN2L 2_57 0.7344 23.58 0.0002246 -4.454 2
3886 SPECC1 17_16 0.7326 23.29 0.0002213 -4.591 1
2760 PDCD10 3_103 0.7122 20.41 0.0001886 -4.030 1
9840 TMEM222 1_19 0.7116 23.53 0.0002172 3.936 2
3391 TBC1D15 12_44 0.6959 22.66 0.0002045 4.461 2
2445 MDK 11_28 0.6770 38.32 0.0003365 -6.344 1
3741 KLC1 14_54 0.6618 41.08 0.0003526 6.933 1
8880 DIRAS1 19_3 0.6540 21.35 0.0001811 4.119 1
8068 INO80E 16_24 0.6482 38.30 0.0003220 6.230 1
1568 KIAA0391 14_9 0.6447 23.53 0.0001967 -4.788 2
8916 LY6H 8_94 0.6192 23.06 0.0001852 4.236 1
5581 FAM134A 2_129 0.5715 23.55 0.0001746 -4.682 2Genes with largest effect sizes
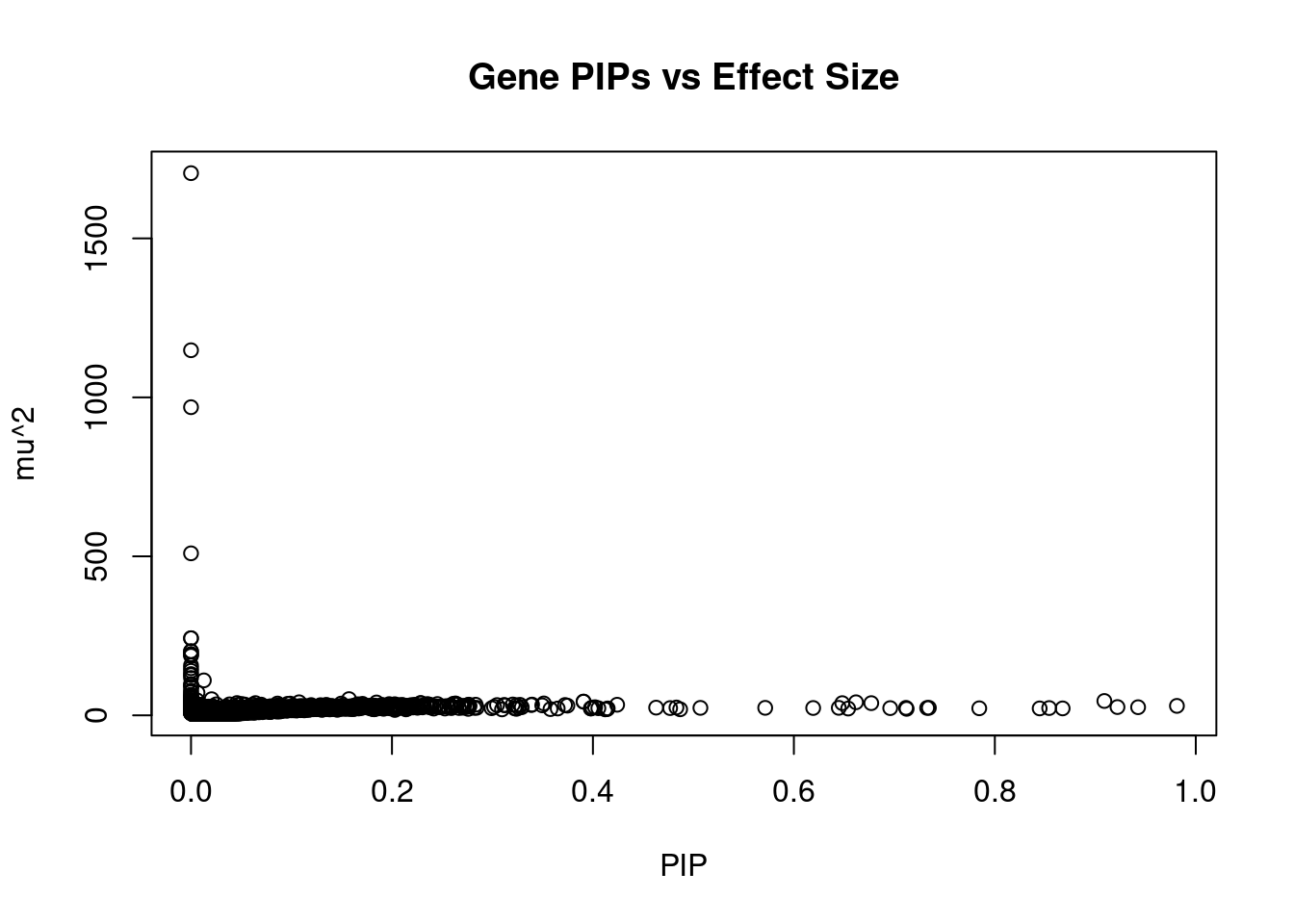
genename region_tag susie_pip mu2 PVE z num_eqtl
12858 HIST1H2BO 6_21 8.042e-11 1705.20 1.779e-12 -7.423 1
9594 HIST1H1B 6_21 1.743e-14 1148.25 2.596e-16 -8.699 1
11457 HIST1H2BN 6_21 1.414e-06 969.11 1.778e-08 10.773 1
6434 MMP16 8_63 0.000e+00 509.49 0.000e+00 3.645 1
11416 HLA-DQB2 6_26 5.551e-16 242.45 1.746e-18 -3.919 1
11576 HLA-DQA2 6_26 5.551e-16 242.45 1.746e-18 -3.919 1
10760 APOM 6_26 1.079e-08 201.55 2.821e-11 8.945 1
10755 ABHD16A 6_26 8.934e-09 201.25 2.332e-11 8.934 1
11740 C4A 6_26 2.019e-10 192.59 5.044e-13 8.445 1
10753 MSH5 6_26 0.000e+00 190.83 0.000e+00 7.722 1
10989 DDAH2 6_26 0.000e+00 187.23 0.000e+00 7.661 1
10266 HLA-DQA1 6_26 0.000e+00 157.40 0.000e+00 -1.344 2
3613 HIST1H2BJ 6_21 0.000e+00 148.31 0.000e+00 1.674 1
10158 HLA-DRB1 6_26 0.000e+00 139.84 0.000e+00 5.148 1
10762 BAG6 6_26 1.110e-16 129.77 1.869e-19 6.613 1
10727 PBX2 6_26 0.000e+00 126.29 0.000e+00 3.355 1
10984 ATF6B 6_26 0.000e+00 117.45 0.000e+00 3.821 1
2073 MPP6 7_21 1.278e-02 109.91 1.822e-05 -3.302 1
10747 C6orf48 6_26 2.220e-16 98.21 2.829e-19 5.387 2
10732 PRRT1 6_26 0.000e+00 95.47 0.000e+00 -7.907 1Genes with highest PVE
genename region_tag susie_pip mu2 PVE z num_eqtl
2890 SF3B1 2_117 0.9090 45.13 0.0005321 6.784 1
10447 ZNF823 19_10 0.9812 29.69 0.0003779 5.485 1
3741 KLC1 14_54 0.6618 41.08 0.0003526 6.933 1
2445 MDK 11_28 0.6770 38.32 0.0003365 -6.344 1
8068 INO80E 16_24 0.6482 38.30 0.0003220 6.230 1
5935 ARFGAP2 11_29 0.9426 25.52 0.0003120 4.839 1
3025 MAP7D1 1_22 0.9221 25.98 0.0003107 5.058 1
8557 MAP3K11 11_36 0.8541 22.88 0.0002535 -4.409 1
11504 AC012074.2 2_15 0.8675 21.80 0.0002452 4.457 2
104 ELAC2 17_11 0.8446 21.79 0.0002387 4.518 1
3216 HSDL2 9_57 0.7845 22.13 0.0002251 4.378 1
2823 LMAN2L 2_57 0.7344 23.58 0.0002246 -4.454 2
3886 SPECC1 17_16 0.7326 23.29 0.0002213 -4.591 1
7005 MAIP1 2_118 0.3907 43.09 0.0002183 7.321 1
7004 TYW5 2_118 0.3907 43.09 0.0002183 -7.321 1
9840 TMEM222 1_19 0.7116 23.53 0.0002172 3.936 2
3391 TBC1D15 12_44 0.6959 22.66 0.0002045 4.461 2
1568 KIAA0391 14_9 0.6447 23.53 0.0001967 -4.788 2
2760 PDCD10 3_103 0.7122 20.41 0.0001886 -4.030 1
8916 LY6H 8_94 0.6192 23.06 0.0001852 4.236 1Genes with largest z scores
genename region_tag susie_pip mu2 PVE z num_eqtl
11457 HIST1H2BN 6_21 1.414e-06 969.11 1.778e-08 10.773 1
10760 APOM 6_26 1.079e-08 201.55 2.821e-11 8.945 1
10755 ABHD16A 6_26 8.934e-09 201.25 2.332e-11 8.934 1
5732 PPP1R18 6_24 3.681e-04 89.59 4.278e-07 8.730 1
9594 HIST1H1B 6_21 1.743e-14 1148.25 2.596e-16 -8.699 1
11740 C4A 6_26 2.019e-10 192.59 5.044e-13 8.445 1
4810 PGBD1 6_22 6.452e-03 71.16 5.955e-06 -8.295 2
9231 HIST1H2BC 6_20 2.051e-02 50.70 1.349e-05 -7.978 1
10732 PRRT1 6_26 0.000e+00 95.47 0.000e+00 -7.907 1
10753 MSH5 6_26 0.000e+00 190.83 0.000e+00 7.722 1
10989 DDAH2 6_26 0.000e+00 187.23 0.000e+00 7.661 1
10729 RNF5 6_26 0.000e+00 89.08 0.000e+00 7.459 2
12858 HIST1H2BO 6_21 8.042e-11 1705.20 1.779e-12 -7.423 1
7005 MAIP1 2_118 3.907e-01 43.09 2.183e-04 7.321 1
7004 TYW5 2_118 3.907e-01 43.09 2.183e-04 -7.321 1
11468 TRIM26 6_24 2.888e-12 64.86 2.430e-15 -7.007 2
3741 KLC1 14_54 6.618e-01 41.08 3.526e-04 6.933 1
9836 BTN3A2 6_20 1.572e-01 51.01 1.040e-04 6.821 1
2890 SF3B1 2_117 9.090e-01 45.13 5.321e-04 6.784 1
10762 BAG6 6_26 1.110e-16 129.77 1.869e-19 6.613 1Comparing z scores and PIPs
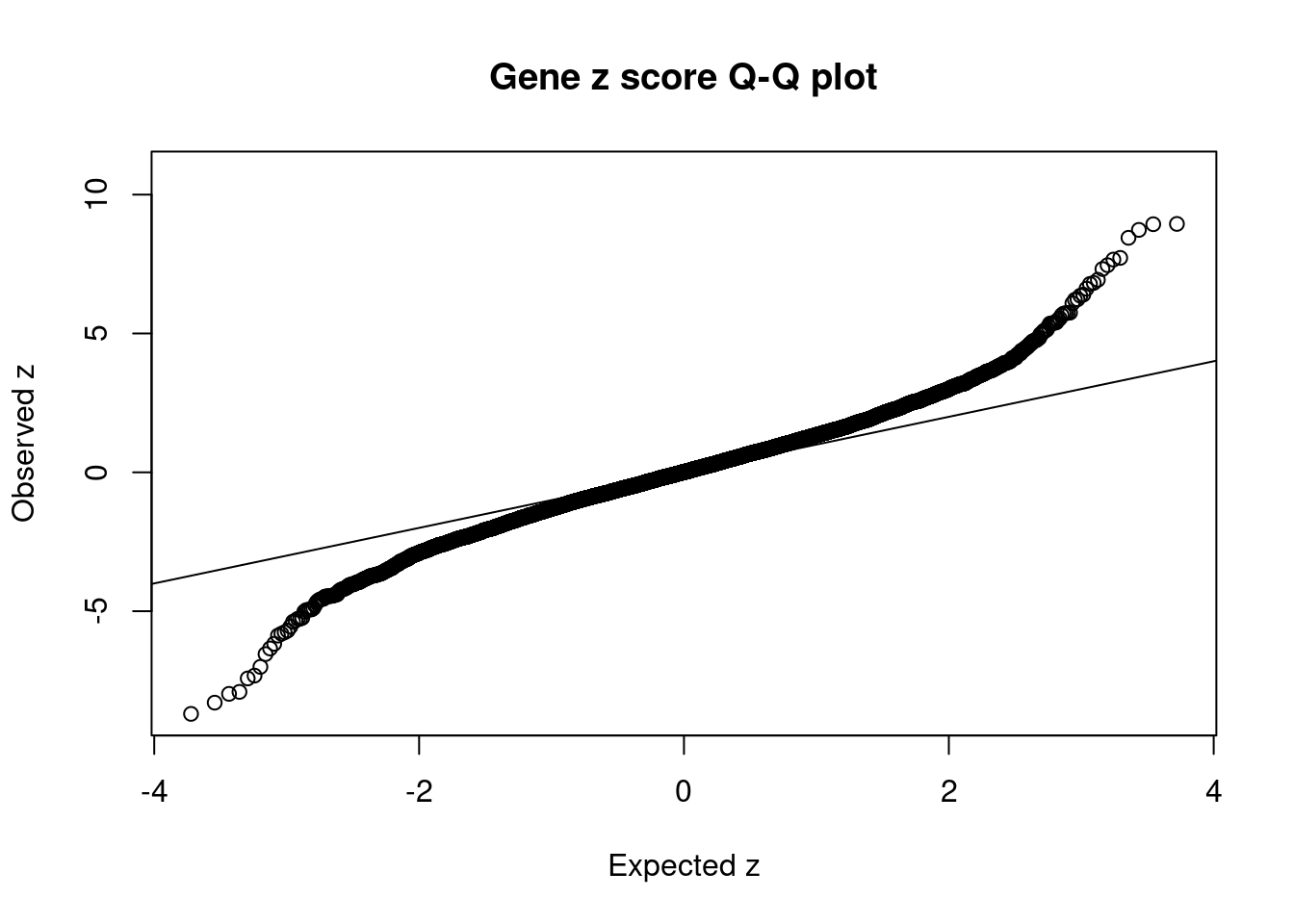
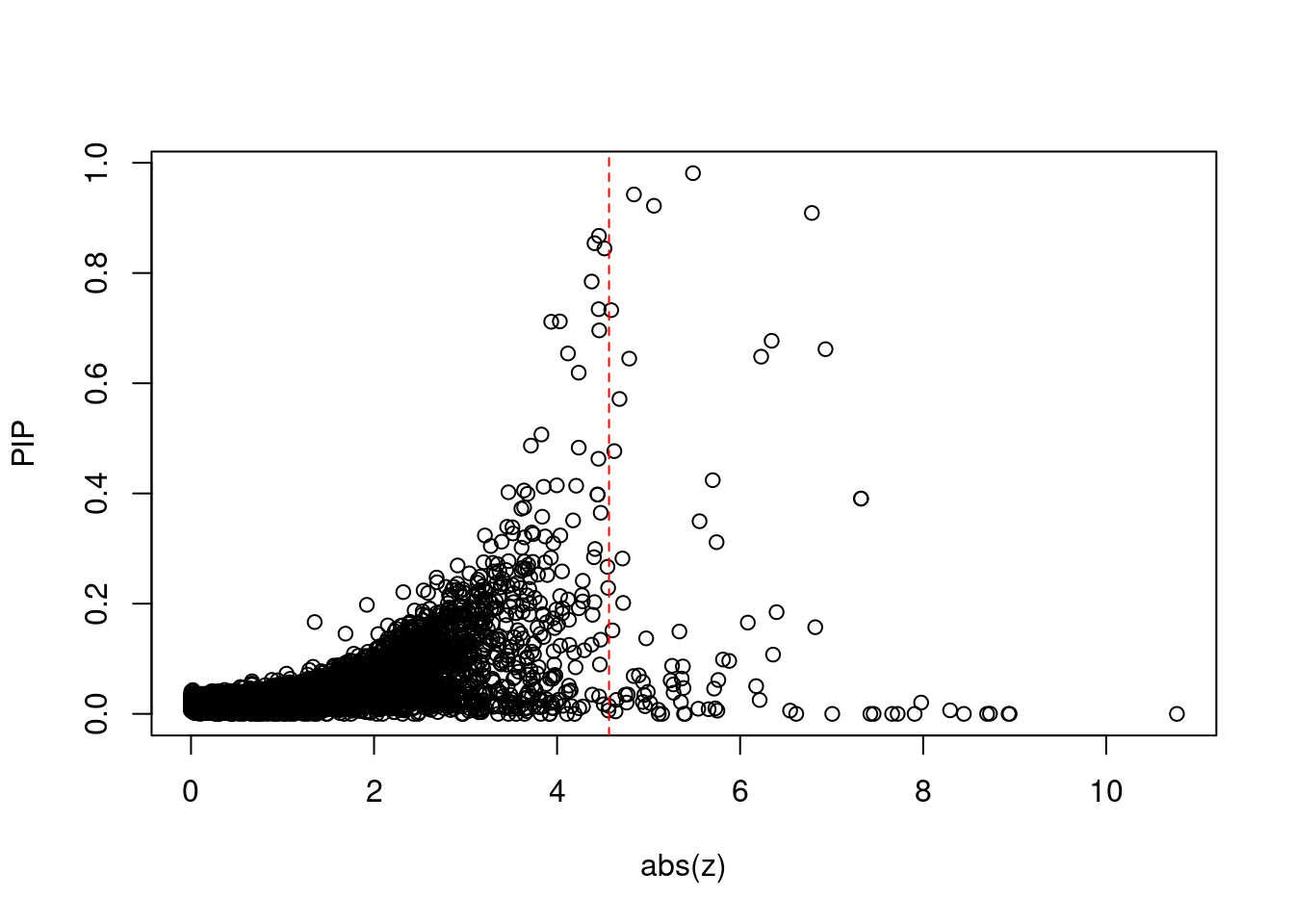
[1] 0.007601GO enrichment analysis for genes with PIP>0.5
#number of genes for gene set enrichment
length(genes)[1] 21Uploading data to Enrichr... Done.
Querying GO_Biological_Process_2021... Done.
Querying GO_Cellular_Component_2021... Done.
Querying GO_Molecular_Function_2021... Done.
Parsing results... Done.
[1] "GO_Biological_Process_2021"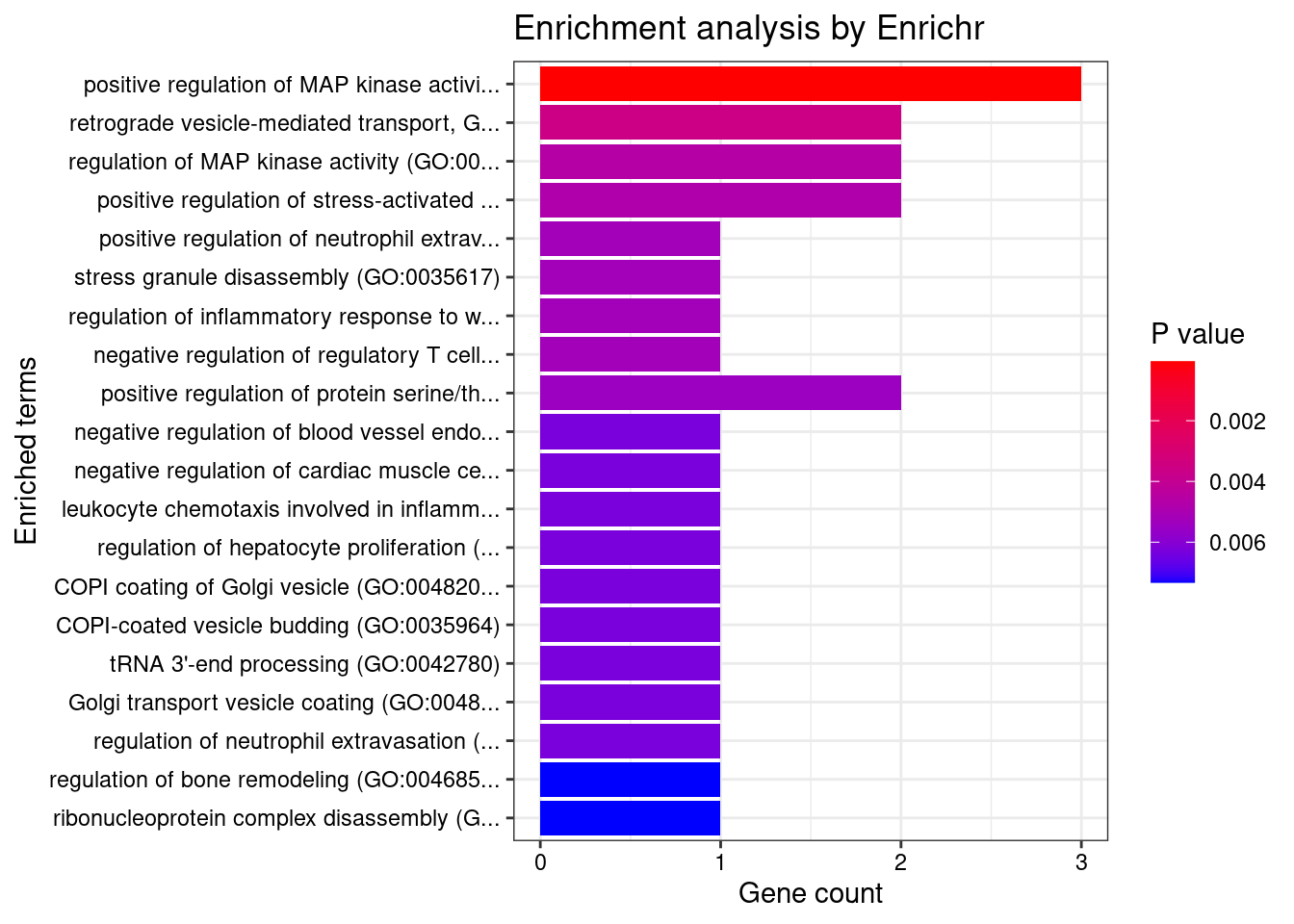
Term Overlap
1 positive regulation of MAP kinase activity (GO:0043406) 3/69
Adjusted.P.value Genes
1 0.0112 PDCD10;DIRAS1;MAP3K11
[1] "GO_Cellular_Component_2021"
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)
[1] "GO_Molecular_Function_2021"
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)DisGeNET enrichment analysis for genes with PIP>0.5
Description FDR Ratio
46 Cerebral Cavernous Malformations 3 0.006714 1/8
47 Reticular Dystrophy Of Retinal Pigment Epithelium 0.006714 1/8
50 Familial cerebral cavernous malformation 0.006714 1/8
53 PROSTATE CANCER, HEREDITARY, 2 0.006714 1/8
55 COMBINED OXIDATIVE PHOSPHORYLATION DEFICIENCY 17 0.006714 1/8
56 MENTAL RETARDATION, AUTOSOMAL RECESSIVE 52 0.006714 1/8
57 RETINAL DYSTROPHY WITH OR WITHOUT EXTRAOCULAR ANOMALIES 0.006714 1/8
38 Refractory anemia with ringed sideroblasts 0.011746 1/8
49 Cavernous Hemangioma of Brain 0.015656 1/8
19 Exudative retinopathy 0.016762 1/8
BgRatio
46 1/9703
47 1/9703
50 1/9703
53 1/9703
55 1/9703
56 1/9703
57 1/9703
38 2/9703
49 3/9703
19 4/9703WebGestalt enrichment analysis for genes with PIP>0.5
Loading the functional categories...
Loading the ID list...
Loading the reference list...
Performing the enrichment analysis...Warning in oraEnrichment(interestGeneList, referenceGeneList, geneSet, minNum =
minNum, : No significant gene set is identified based on FDR 0.05!NULLPIP Manhattan Plot
Warning: 'timedatectl' indicates the non-existent timezone name 'n/a'Warning: Your system is mis-configured: '/etc/localtime' is not a symlinkWarning: It is strongly recommended to set envionment variable TZ to 'America/
Chicago' (or equivalent)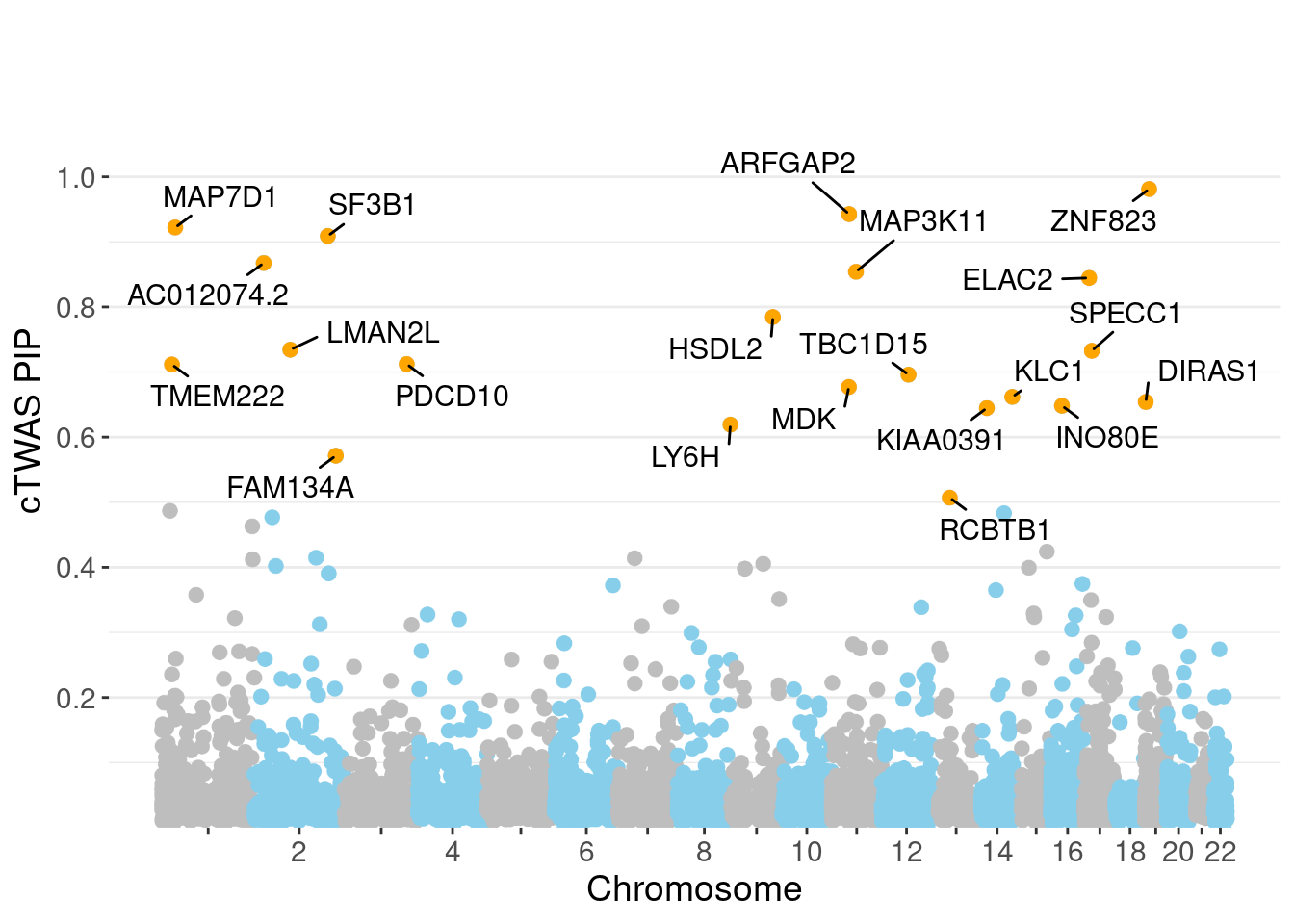
Sensitivity, specificity and precision for silver standard genes
#number of genes in known annotations
print(length(known_annotations))[1] 130#number of genes in known annotations with imputed expression
print(sum(known_annotations %in% ctwas_gene_res$genename))[1] 57#significance threshold for TWAS
print(sig_thresh)[1] 4.567#number of ctwas genes
length(ctwas_genes)[1] 7#number of TWAS genes
length(twas_genes)[1] 77#show novel genes (ctwas genes with not in TWAS genes)
ctwas_gene_res[ctwas_gene_res$genename %in% novel_genes,report_cols] genename region_tag susie_pip mu2 PVE z num_eqtl
11504 AC012074.2 2_15 0.8675 21.80 0.0002452 4.457 2
8557 MAP3K11 11_36 0.8541 22.88 0.0002535 -4.409 1
104 ELAC2 17_11 0.8446 21.79 0.0002387 4.518 1#sensitivity / recall
print(sensitivity) ctwas TWAS
0.02308 0.04615 #specificity
print(specificity) ctwas TWAS
0.9996 0.9930 #precision / PPV
print(precision) ctwas TWAS
0.42857 0.07792 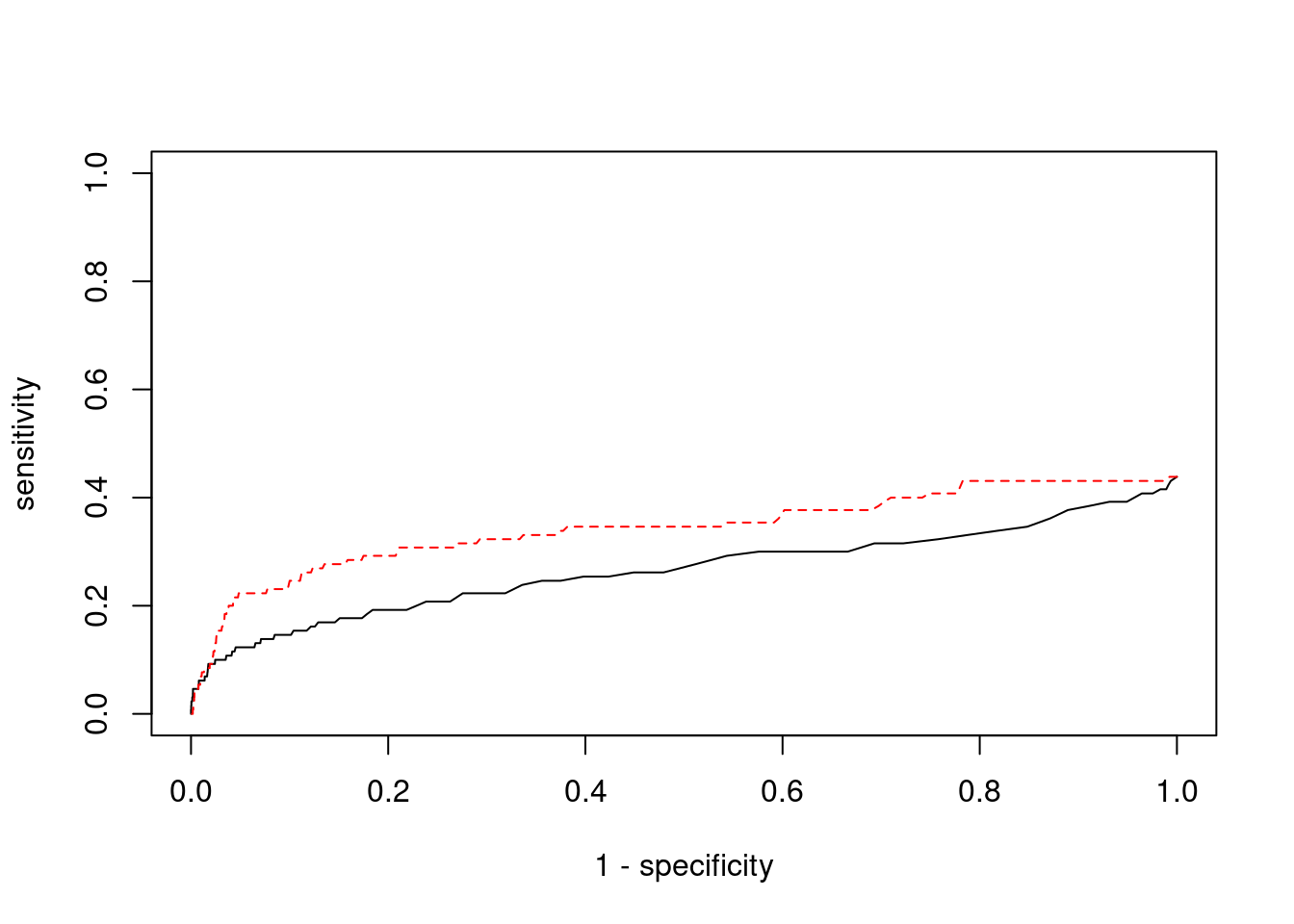
cTWAS is more precise than TWAS in distinguishing silver standard and bystander genes
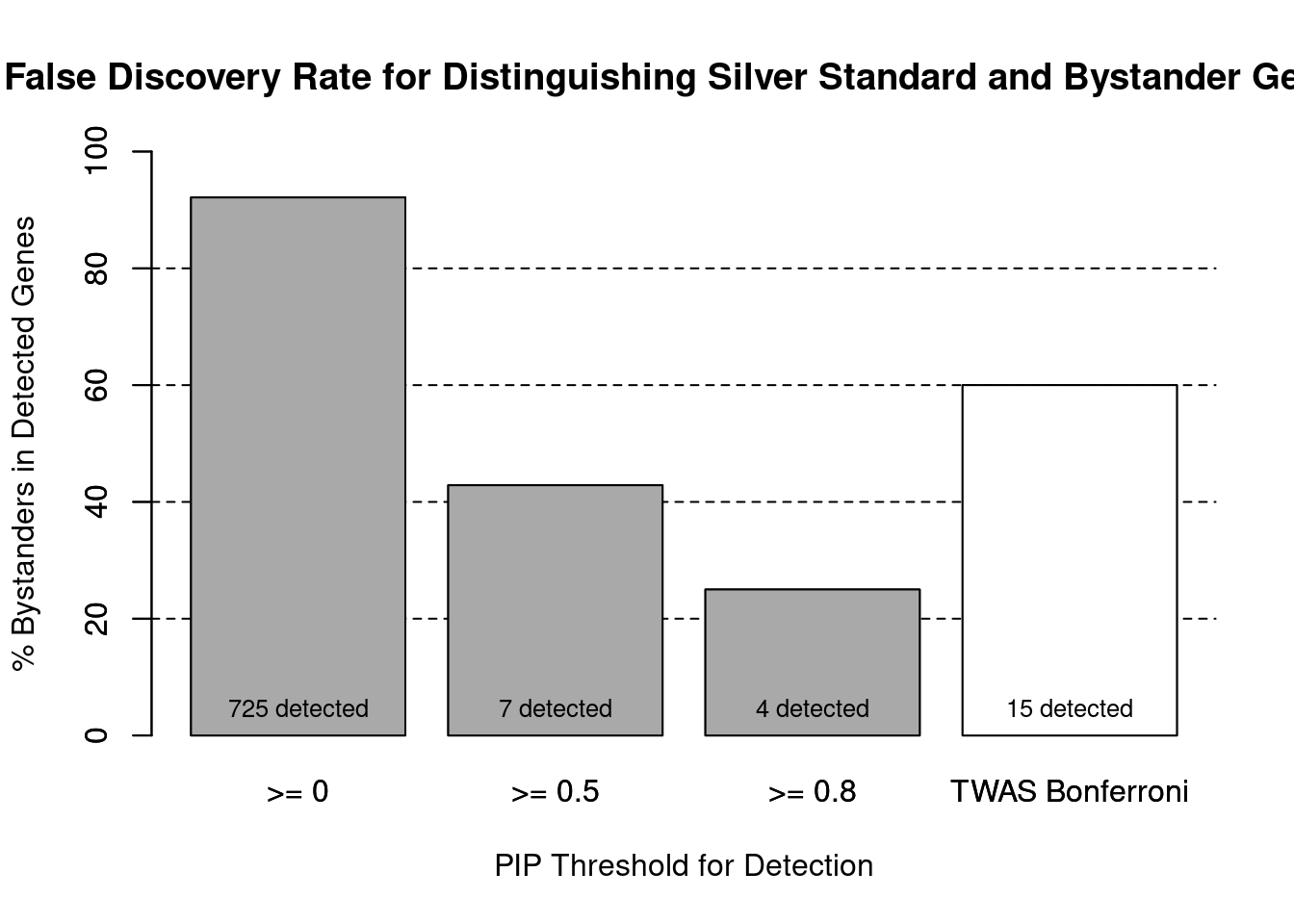
#number of genes in known annotations (with imputed expression)
print(length(known_annotations))[1] 57#number of bystander genes (with imputed expression)
print(length(unrelated_genes))[1] 667#subset results to genes in known annotations or bystanders
ctwas_gene_res_subset <- ctwas_gene_res[ctwas_gene_res$genename %in% c(known_annotations, unrelated_genes),]
#assign ctwas and TWAS genes
ctwas_genes <- ctwas_gene_res_subset$genename[ctwas_gene_res_subset$susie_pip>0.8]
twas_genes <- ctwas_gene_res_subset$genename[abs(ctwas_gene_res_subset$z)>sig_thresh]
#significance threshold for TWAS
print(sig_thresh)[1] 4.567#number of ctwas genes (in known annotations or bystanders)
length(ctwas_genes)[1] 4#number of TWAS genes (in known annotations or bystanders)
length(twas_genes)[1] 15#sensitivity / recall
sensitivity ctwas TWAS
0.05263 0.10526 #specificity / (1 - False Positive Rate)
specificity ctwas TWAS
0.9985 0.9865 #precision / PPV / (1 - False Discovery Rate)
precisionctwas TWAS
0.75 0.40 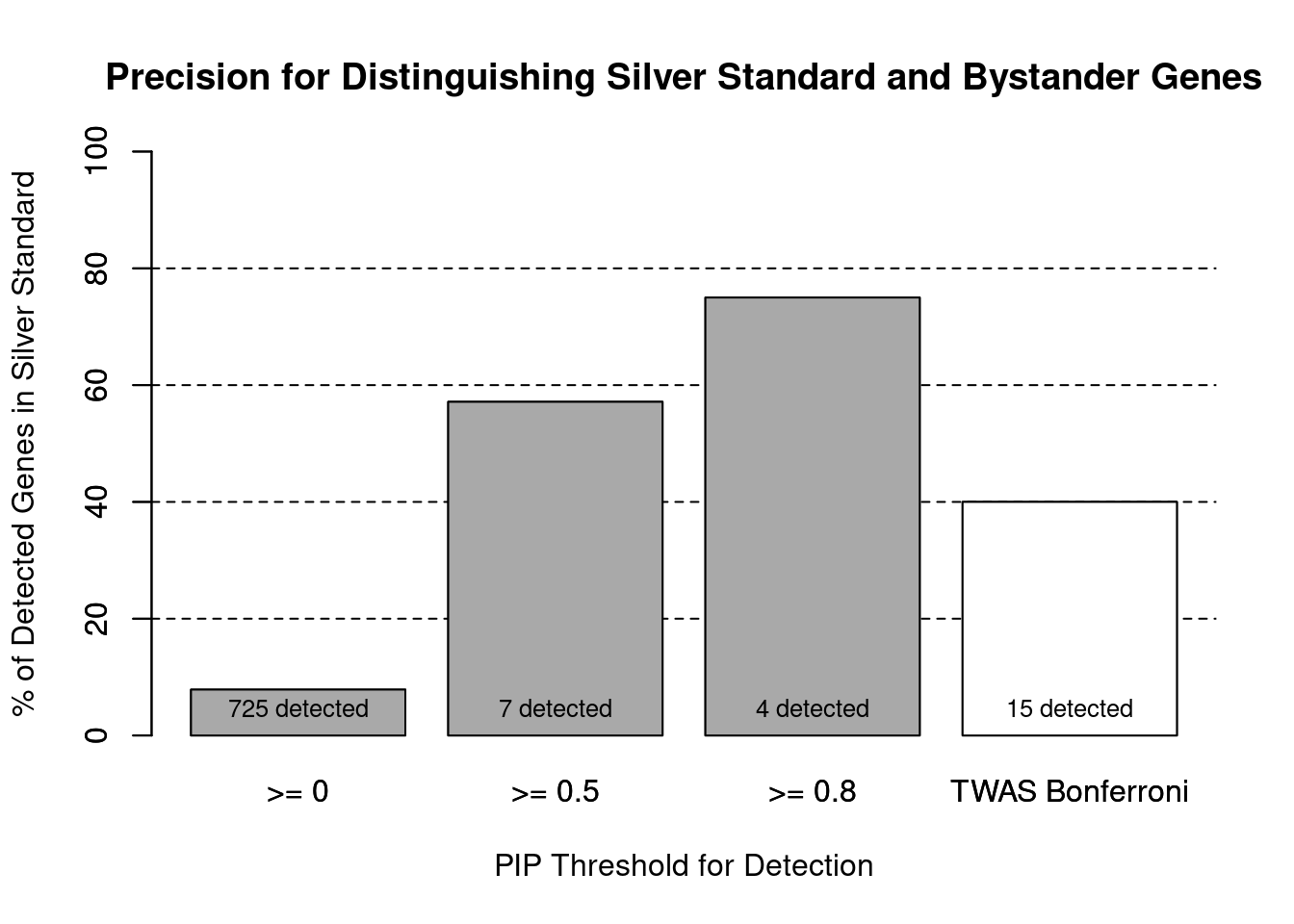
pip_range <- (0:1000)/1000
sensitivity <- rep(NA, length(pip_range))
specificity <- rep(NA, length(pip_range))
for (index in 1:length(pip_range)){
pip <- pip_range[index]
ctwas_genes <- ctwas_gene_res_subset$genename[ctwas_gene_res_subset$susie_pip>=pip]
sensitivity[index] <- sum(ctwas_genes %in% known_annotations)/length(known_annotations)
specificity[index] <- sum(!(unrelated_genes %in% ctwas_genes))/length(unrelated_genes)
}
plot(1-specificity, sensitivity, type="l", xlim=c(0,1), ylim=c(0,1), main="", xlab="1 - Specificity", ylab="Sensitivity")
title(expression("ROC Curve for cTWAS (black) and TWAS (" * phantom("red") * ")"))
title(expression(phantom("ROC Curve for cTWAS (black) and TWAS (") * "red" * phantom(")")), col.main="red")
sig_thresh_range <- seq(from=0, to=max(abs(ctwas_gene_res_subset$z)), length.out=length(pip_range))
for (index in 1:length(sig_thresh_range)){
sig_thresh_plot <- sig_thresh_range[index]
twas_genes <- ctwas_gene_res_subset$genename[abs(ctwas_gene_res_subset$z)>=sig_thresh_plot]
sensitivity[index] <- sum(twas_genes %in% known_annotations)/length(known_annotations)
specificity[index] <- sum(!(unrelated_genes %in% twas_genes))/length(unrelated_genes)
}
lines(1-specificity, sensitivity, xlim=c(0,1), ylim=c(0,1), col="red", lty=1)
abline(a=0,b=1,lty=3)
#add previously computed points from the analysis
ctwas_genes <- ctwas_gene_res_subset$genename[ctwas_gene_res_subset$susie_pip>0.8]
twas_genes <- ctwas_gene_res_subset$genename[abs(ctwas_gene_res_subset$z)>sig_thresh]
points(1-specificity_plot["ctwas"], sensitivity_plot["ctwas"], pch=21, bg="black")
points(1-specificity_plot["TWAS"], sensitivity_plot["TWAS"], pch=21, bg="red")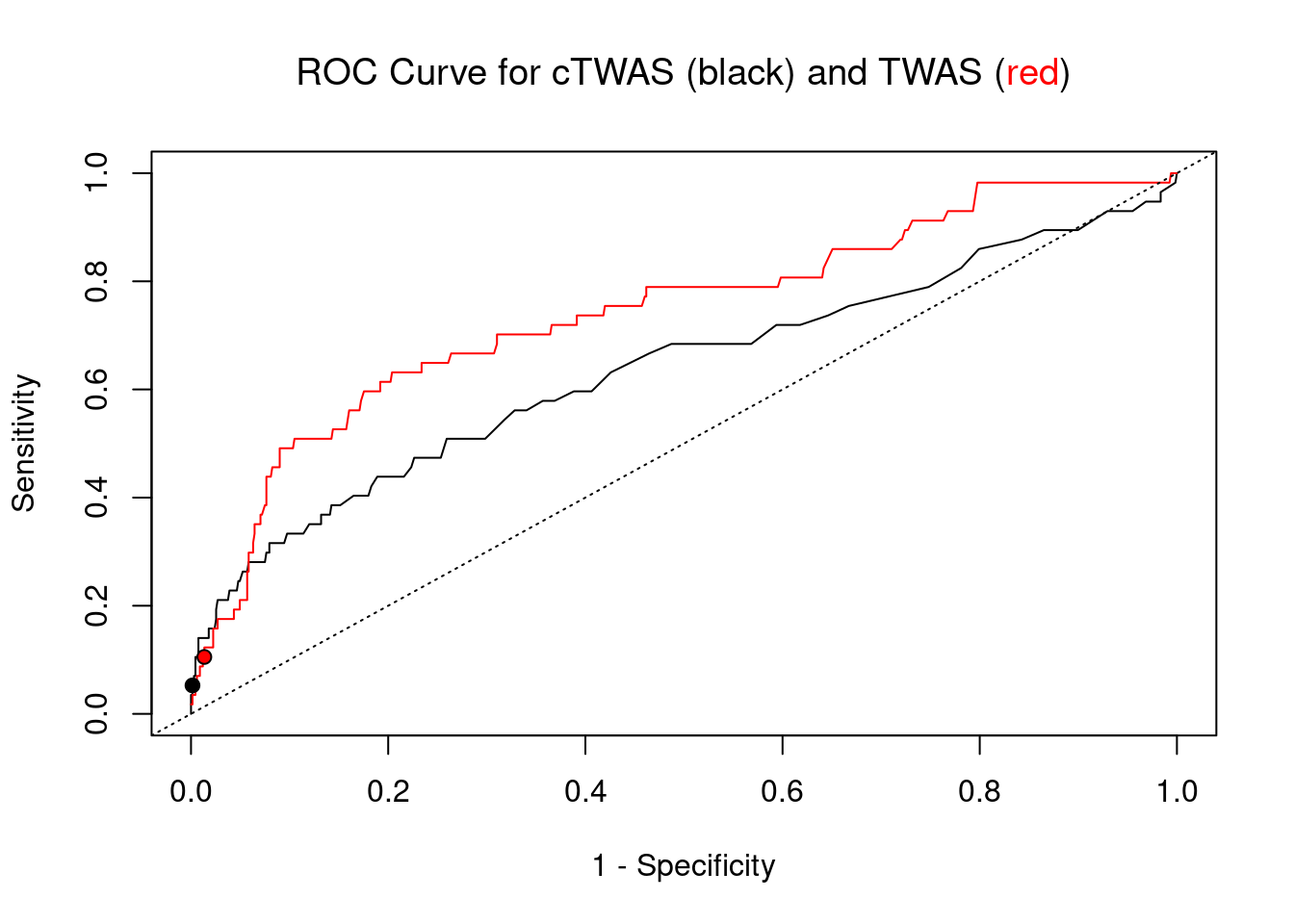
Undetected silver standard genes have low TWAS z-scores or stronger signal from nearby variants
#table of outcomes for silver standard genes
-sort(-table(silver_standard_case))silver_standard_case
Not Imputed Insignificant z-score Nearby SNP(s)
73 50 4
Detected (PIP > 0.8)
3 #show inconclusive genes
silver_standard_case[silver_standard_case=="Inconclusive"]named character(0)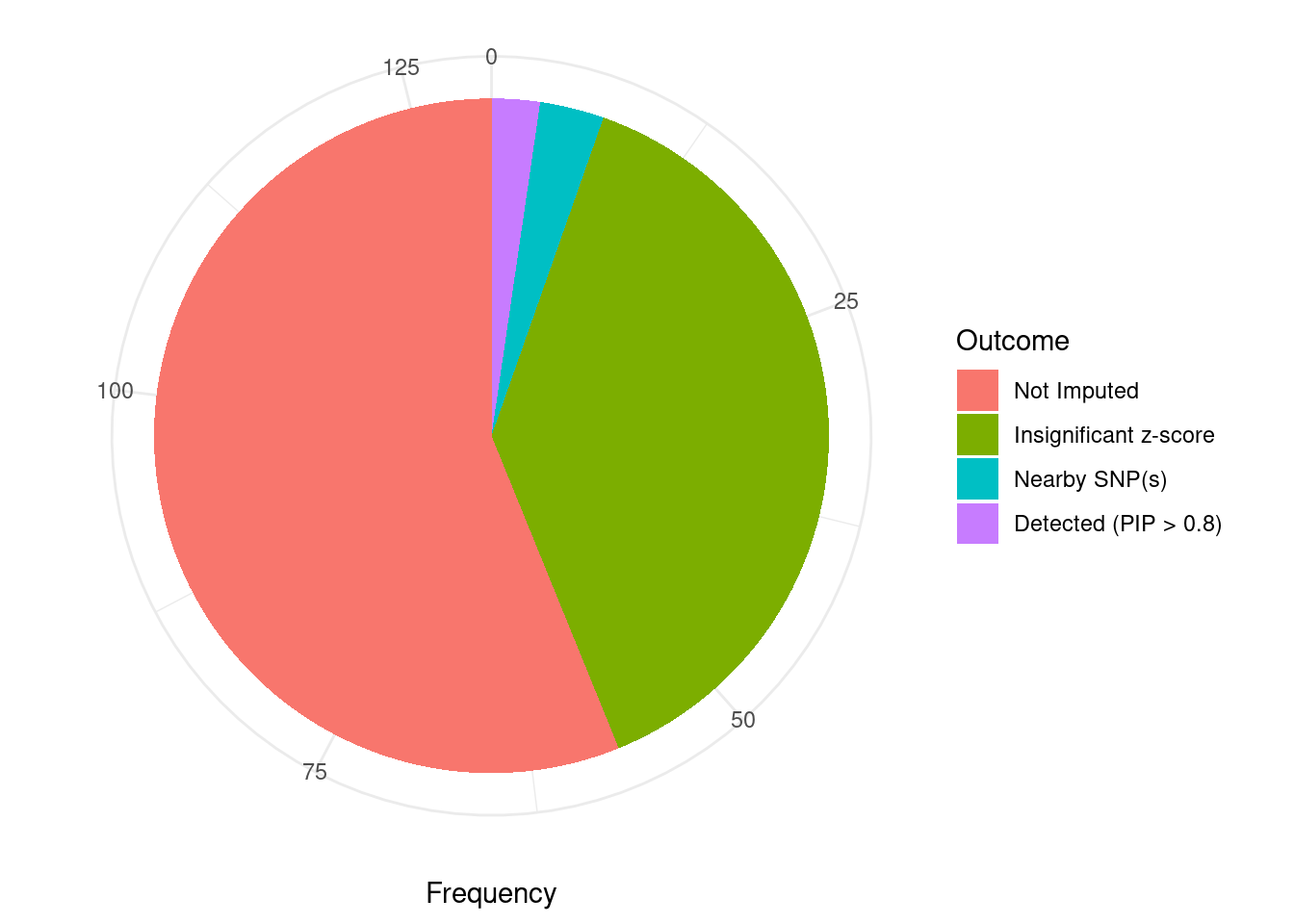
sessionInfo()R version 3.6.1 (2019-07-05)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Scientific Linux 7.4 (Nitrogen)
Matrix products: default
BLAS/LAPACK: /software/openblas-0.2.19-el7-x86_64/lib/libopenblas_haswellp-r0.2.19.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] parallel stats4 stats graphics grDevices utils datasets
[8] methods base
other attached packages:
[1] GenomicRanges_1.36.1 GenomeInfoDb_1.20.0 IRanges_2.18.1
[4] S4Vectors_0.22.1 BiocGenerics_0.30.0 biomaRt_2.40.1
[7] readxl_1.3.1 forcats_0.5.1 stringr_1.4.0
[10] dplyr_1.0.7 purrr_0.3.4 readr_2.1.1
[13] tidyr_1.1.4 tidyverse_1.3.1 tibble_3.1.6
[16] WebGestaltR_0.4.4 disgenet2r_0.99.2 enrichR_3.0
[19] cowplot_1.0.0 ggplot2_3.3.5 workflowr_1.7.0
loaded via a namespace (and not attached):
[1] ggbeeswarm_0.6.0 colorspace_2.0-2 rjson_0.2.20
[4] ellipsis_0.3.2 rprojroot_2.0.2 XVector_0.24.0
[7] fs_1.5.2 rstudioapi_0.13 farver_2.1.0
[10] ggrepel_0.9.1 bit64_4.0.5 AnnotationDbi_1.46.0
[13] fansi_1.0.2 lubridate_1.8.0 xml2_1.3.3
[16] codetools_0.2-16 doParallel_1.0.17 cachem_1.0.6
[19] knitr_1.36 jsonlite_1.7.2 apcluster_1.4.8
[22] Cairo_1.5-12.2 broom_0.7.10 dbplyr_2.1.1
[25] compiler_3.6.1 httr_1.4.2 backports_1.4.1
[28] assertthat_0.2.1 Matrix_1.2-18 fastmap_1.1.0
[31] cli_3.1.0 later_0.8.0 prettyunits_1.1.1
[34] htmltools_0.5.2 tools_3.6.1 igraph_1.2.10
[37] GenomeInfoDbData_1.2.1 gtable_0.3.0 glue_1.6.2
[40] reshape2_1.4.4 doRNG_1.8.2 Rcpp_1.0.8
[43] Biobase_2.44.0 cellranger_1.1.0 jquerylib_0.1.4
[46] vctrs_0.3.8 svglite_1.2.2 iterators_1.0.14
[49] xfun_0.29 ps_1.6.0 rvest_1.0.2
[52] lifecycle_1.0.1 rngtools_1.5.2 XML_3.99-0.3
[55] zlibbioc_1.30.0 getPass_0.2-2 scales_1.1.1
[58] vroom_1.5.7 hms_1.1.1 promises_1.0.1
[61] yaml_2.2.1 curl_4.3.2 memoise_2.0.1
[64] ggrastr_1.0.1 gdtools_0.1.9 stringi_1.7.6
[67] RSQLite_2.2.8 highr_0.9 foreach_1.5.2
[70] rlang_1.0.1 pkgconfig_2.0.3 bitops_1.0-7
[73] evaluate_0.14 lattice_0.20-38 labeling_0.4.2
[76] bit_4.0.4 processx_3.5.2 tidyselect_1.1.1
[79] plyr_1.8.6 magrittr_2.0.2 R6_2.5.1
[82] generics_0.1.1 DBI_1.1.2 pillar_1.6.4
[85] haven_2.4.3 whisker_0.3-2 withr_2.4.3
[88] RCurl_1.98-1.5 modelr_0.1.8 crayon_1.5.0
[91] utf8_1.2.2 tzdb_0.2.0 rmarkdown_2.11
[94] progress_1.2.2 grid_3.6.1 data.table_1.14.2
[97] blob_1.2.2 callr_3.7.0 git2r_0.26.1
[100] reprex_2.0.1 digest_0.6.29 httpuv_1.5.1
[103] munsell_0.5.0 beeswarm_0.2.3 vipor_0.4.5